Your Standard electrode potential symbol images are ready. Standard electrode potential symbol are a topic that is being searched for and liked by netizens today. You can Find and Download the Standard electrode potential symbol files here. Download all free photos and vectors.
If you’re looking for standard electrode potential symbol images information related to the standard electrode potential symbol topic, you have come to the ideal blog. Our website frequently provides you with hints for viewing the maximum quality video and image content, please kindly hunt and find more informative video articles and images that match your interests.
Standard Electrode Potential Symbol. It can act as anode half - cell as well as cathode half-cell. Significance of Standard Electrode Potential All electrochemical cells are based on redox reactions which are made up of two half-reactions. All E values are independent of the stoichiometric coefficients. The Standard Hydrogen Electrode The potential of all electrodes are measured by comparing their potential to that of the standard hydrogen electrode.
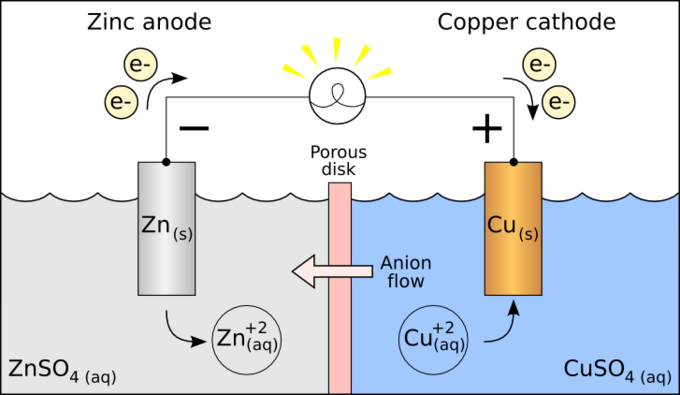 Standard Reduction Potentials Boundless Chemistry From courses.lumenlearning.com
Standard Reduction Potentials Boundless Chemistry From courses.lumenlearning.com
It can act as anode half - cell as well as cathode half-cell. All E values are independent of the stoichiometric coefficients. The standard cell potential is a measure of the driving force for a given redox reaction. Value of its standard reduction potential and standard oxidation potential is always zero at 25 or 298K. The Standard Hydrogen Electrode The potential of all electrodes are measured by comparing their potential to that of the standard hydrogen electrode. Ag e Ag.
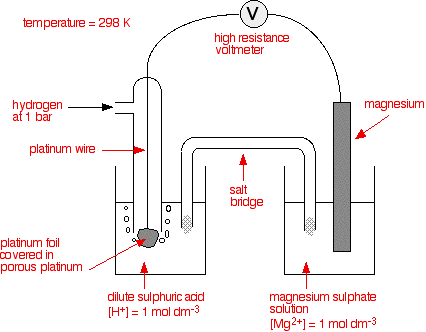
A temperature or 298K 1 atmosphere pressure.
The standard hydrogen electrode SHE is assigned the potential of 0 volts. Of a cell is given the symbol E if this potential is standard It is measured by using standard hydrogen electrode in this case the potential is given the symbol Eº. The potential of the standard hydrogen electrode SHE is defined as 0 V under standard conditions. In electrochemistry the standard electrode potential abbreviated E or E is the measure of individual potential of a reversible electrode at standard state which is with solutes at an effective concentration of 1 mol dm3 and gases at a pressure of 1 atm. It is denoted by the symbol E. Ag e Ag.
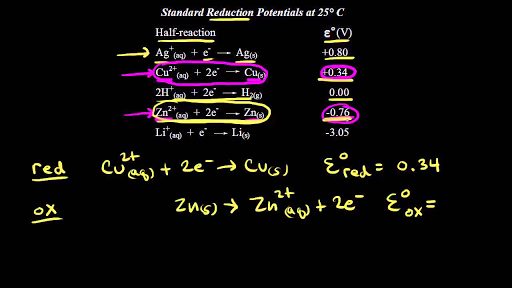
With respect to SHE oxidation occurs and it will given negative sign. The electrode potential at standard conditions such as 25C temperature 1 atm pressure 1 M concentration of electrolyte is called the standard electrode potential. For example the standard potential of the Zn2 Zn electrode denoted EZn2 Zn is the emf of the cell in which the reaction Zn2aq H 2 2H aq Zn takes. A temperature or 298K 1 atmosphere pressure. Standard hydrogen electrode is a gas ion electrode.

With respect to SHE oxidation occurs and it will given negative sign. The reduction potential is an intensive property. All E values are independent of the stoichiometric coefficients. H2 g 2H aq 2e-Because the equilibrium does not include a. In the half cell the metal electrode is suspended in a solution of one molar concentration at 298rmK and the electrode potential is called standard electrode potential.
 Source: nagwa.com
Source: nagwa.com
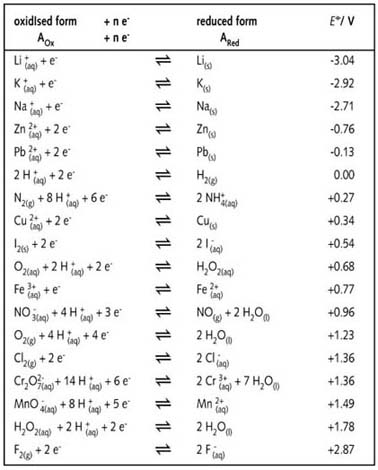
The symbol E o cell represents the standard electrode potential of a cell. It can act as anode half - cell as well as cathode half-cell. 2 E o is measured in volts V. Standard electrode potential data page and Caesium See more. Standard Electrode Half-Cell Potentials.

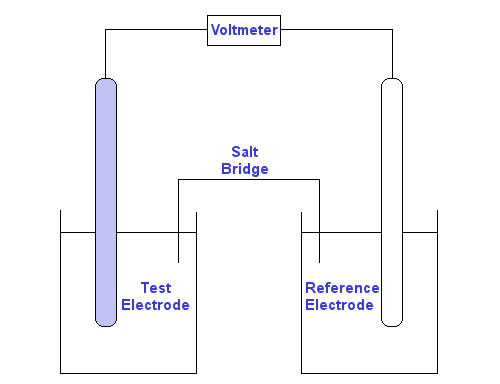
The electrical potential of the standard half-cell determines both the magnitude and sign of the standard half-cell potential. In the half cell the metal electrode is suspended in a solution of one molar concentration at 298rmK and the electrode potential is called standard electrode potential. Standard electrode potential In electrochemistry the standard electrode potential abbreviated Eo E0 or EO with a superscript plimsoll character pronounced nought is the measure of individual potential of a reversible electrode at equilibrium at standard state. The potential of the standard hydrogen electrode SHE is defined as 0 V under standard conditions. All E values are independent of the stoichiometric coefficients.
 Source: brainkart.com
Source: brainkart.com
If the process that occurs in the half-cell reduces a solution species or the electrode material electrons traverse the external circuit toward the half-cell. It can act as anode half - cell as well as cathode half-cell. With respect to SHE oxidation occurs and it will given negative sign. AgCl e Ag Cl. H2 g 2H aq 2e-Because the equilibrium does not include a.
 Source: slideplayer.com
Source: slideplayer.com
Standard hydrogen electrode is a gas ion electrode. E V Ag e Ag. If the process that occurs in the half-cell reduces a solution species or the electrode material electrons traverse the external circuit toward the half-cell. AgCl e Ag Cl. The electrode potential at standard conditions such as 25C temperature 1 atm pressure 1 M concentration of electrolyte is called the standard electrode potential.
 Source: kenyaplex.com
Source: kenyaplex.com
Standard Electrode Half-Cell Potentials. 2 E o is measured in volts V. The value of oxidation potential of any electrode is equal to negative the value of reduction potential of the same electrode EMF. L Standard Electrode Half-Cell Potentials - Chemistry 2e OpenStax. The standard electrode potential of a metal is the potantial acquired when the metal is immersed in a 1 mol dm -3 solution of its ions at a temperature of 25 o C.
 Source: sciencedirect.com
Source: sciencedirect.com
E V Ag e Ag. Standard electrode potential In electrochemistry the standard electrode potential abbreviated Eo E0 or EO with a superscript plimsoll character pronounced nought is the measure of individual potential of a reversible electrode at equilibrium at standard state. The Standard Hydrogen Electrode The potential of all electrodes are measured by comparing their potential to that of the standard hydrogen electrode. Values of standard electrode potentials are tabulated for reactions in which the reactants and products are in their standard states. The standard cell potential is a measure of the driving force for a given redox reaction.
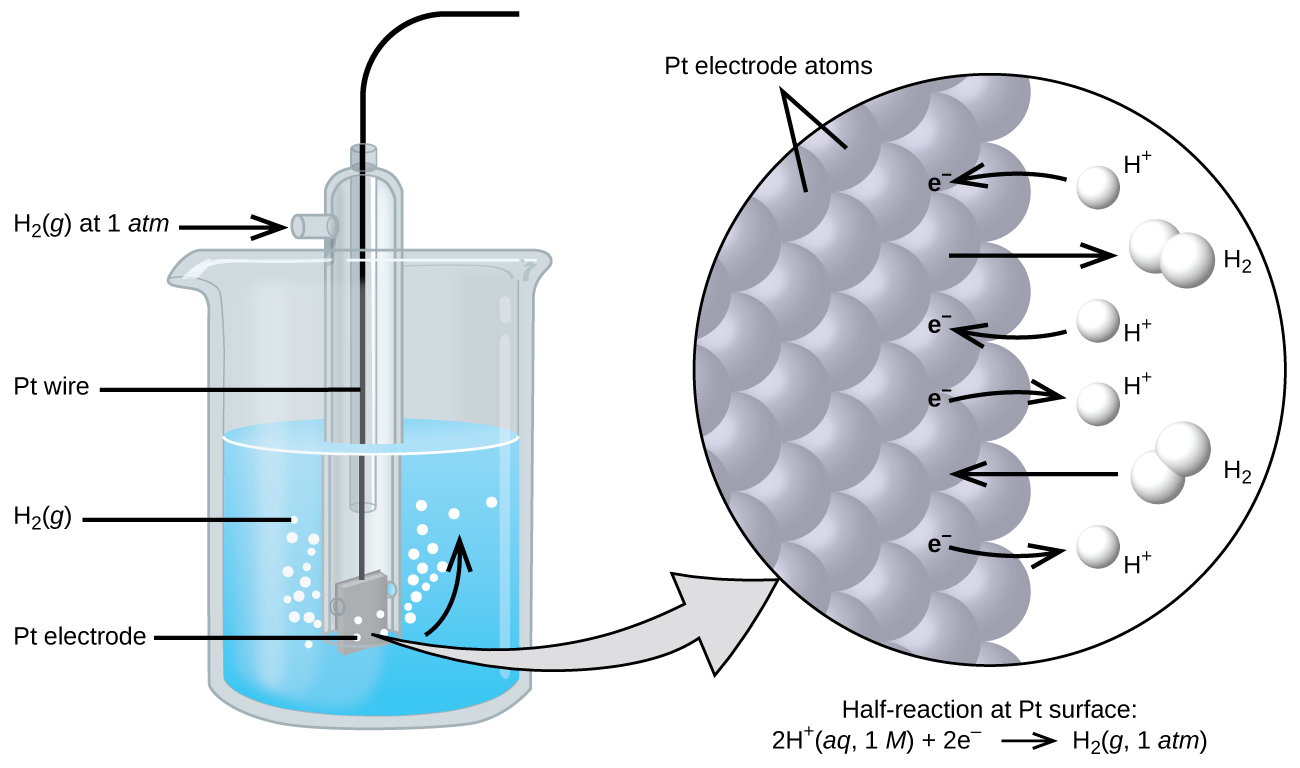 Source: opentextbc.ca
Source: opentextbc.ca
The potential of the standard hydrogen electrode SHE is defined as 0 V under standard conditions. 9 Standard potential of an electrode reaction abbreviated as standard electrode potential is the value of the standard emf of a cell in which molecular hydrogen is oxidized to solvated protons at the left-hand electrode. When zinc electrode is connected with SHE oxidation. The standard electrode potential of a metal is the potantial acquired when the metal is immersed in a 1 mol dm -3 solution of its ions at a temperature of 25 o C. The electrode attached to SHE act as an anode and oxidation takes place on the anode.
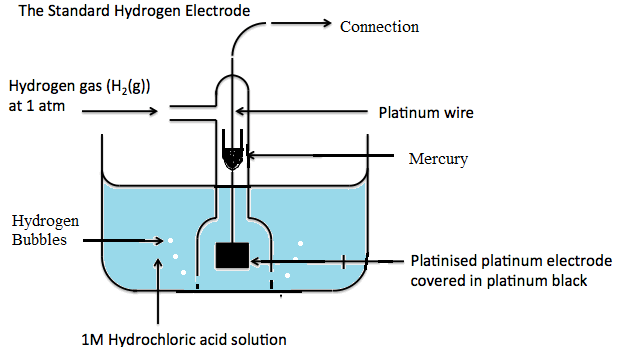 Source: online-sciences.com
Source: online-sciences.com
It is denoted by the symbol E. By the convention the. Standard electrode potential data page and Cadmium See more Caesium Caesium British spelling and IUPAC spelling or cesium American spelling is a chemical element with symbol Cs and atomic number 55. An electrode potential measured under standard conditions. Of a cell is given the symbol E if this potential is standard It is measured by using standard hydrogen electrode in this case the potential is given the symbol Eº.
 Source: chemguide.co.uk
Source: chemguide.co.uk
It is denoted by the symbol E. Value of its standard reduction potential and standard oxidation potential is always zero at 25 or 298K. With respect to SHE oxidation occurs and it will given negative sign. It is used as a reference electrode for determination of standard electrode potential of elements and other half cells. The symbol E o cell represents the standard electrode potential of a cell.

9 Standard potential of an electrode reaction abbreviated as standard electrode potential is the value of the standard emf of a cell in which molecular hydrogen is oxidized to solvated protons at the left-hand electrode. The electrode attached to SHE act as an anode and oxidation takes place on the anode. Standard electrode potential is given negative value when the electrode is combined with standard hydrogen electrode SHE to give a Galvanic cell. 2 E o is measured in volts V. By the convention the.
 Source: ibchem.com
Source: ibchem.com
AgCl e Ag Cl. With respect to SHE oxidation occurs and it will given negative sign. Standard Electrode Half-Cell Potentials. When zinc electrode is connected with SHE oxidation. An electrode potential measured under standard conditions.
 Source: sites.google.com
Source: sites.google.com
Hence the electrical sign of the half-cell terminal is positive. By the convention the. The standard hydrogen electrode SHE is assigned the potential of 0 volts. Standard hydrogen electrode is a gas ion electrode. It has the symbol Eθ and the units V.
 Source: youtube.com
Source: youtube.com
The standard electrode potential of a metal is the potantial acquired when the metal is immersed in a 1 mol dm -3 solution of its ions at a temperature of 25 o C. The electrode potential at standard conditions such as 25C temperature 1 atm pressure 1 M concentration of electrolyte is called the standard electrode potential. Standard electrode potential data page and Caesium See more. The standard hydrogen electrode SHE is assigned the potential of 0 volts. If the process that occurs in the half-cell reduces a solution species or the electrode material electrons traverse the external circuit toward the half-cell.

If the process that occurs in the half-cell reduces a solution species or the electrode material electrons traverse the external circuit toward the half-cell. It is denoted by the symbol E. Video shows what standard electrode potential means. With respect to SHE oxidation occurs and it will given negative sign. Standard hydrogen electrode is a gas ion electrode.
 Source: old.iupac.org
Source: old.iupac.org
Ag e Ag. Standard electrode potential data page and Cadmium See more Caesium Caesium British spelling and IUPAC spelling or cesium American spelling is a chemical element with symbol Cs and atomic number 55. In the half cell the metal electrode is suspended in a solution of one molar concentration at 298rmK and the electrode potential is called standard electrode potential. It is denoted by the symbol E. Significance of Standard Electrode Potential All electrochemical cells are based on redox reactions which are made up of two half-reactions.
 Source: chemicool.com
Source: chemicool.com
The potential of a half-reaction measured against the SHE under standard conditions is called its standard electrode potential. In electrochemistry the standard electrode potential abbreviated E or E is the measure of individual potential of a reversible electrode at standard state which is with solutes at an effective concentration of 1 mol dm3 and gases at a pressure of 1 atm. The standard cell potential is a measure of the driving force for a given redox reaction. Video shows what standard electrode potential means. Standard hydrogen electrode is a gas ion electrode.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title standard electrode potential symbol by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






