Your Molar heat of combustion symbol images are available in this site. Molar heat of combustion symbol are a topic that is being searched for and liked by netizens now. You can Download the Molar heat of combustion symbol files here. Get all free photos.
If you’re looking for molar heat of combustion symbol pictures information related to the molar heat of combustion symbol keyword, you have pay a visit to the right site. Our site frequently gives you suggestions for seeking the highest quality video and picture content, please kindly surf and locate more informative video articles and graphics that fit your interests.
Molar Heat Of Combustion Symbol. 85 rows The heat of combustion of ethanol ΔH c C 2 H 6 O l 239351 6142915. The value corresponds to an exothermic reaction a negative change in. The energy released when one mole of a substance is burned in excess oxygen or air under standard conditions. The molar heat of vaporization equation looks like this.
 Molar Heat Of Combustion Definition Calculations Video Lesson Transcript Study Com From study.com
Molar Heat Of Combustion Definition Calculations Video Lesson Transcript Study Com From study.com
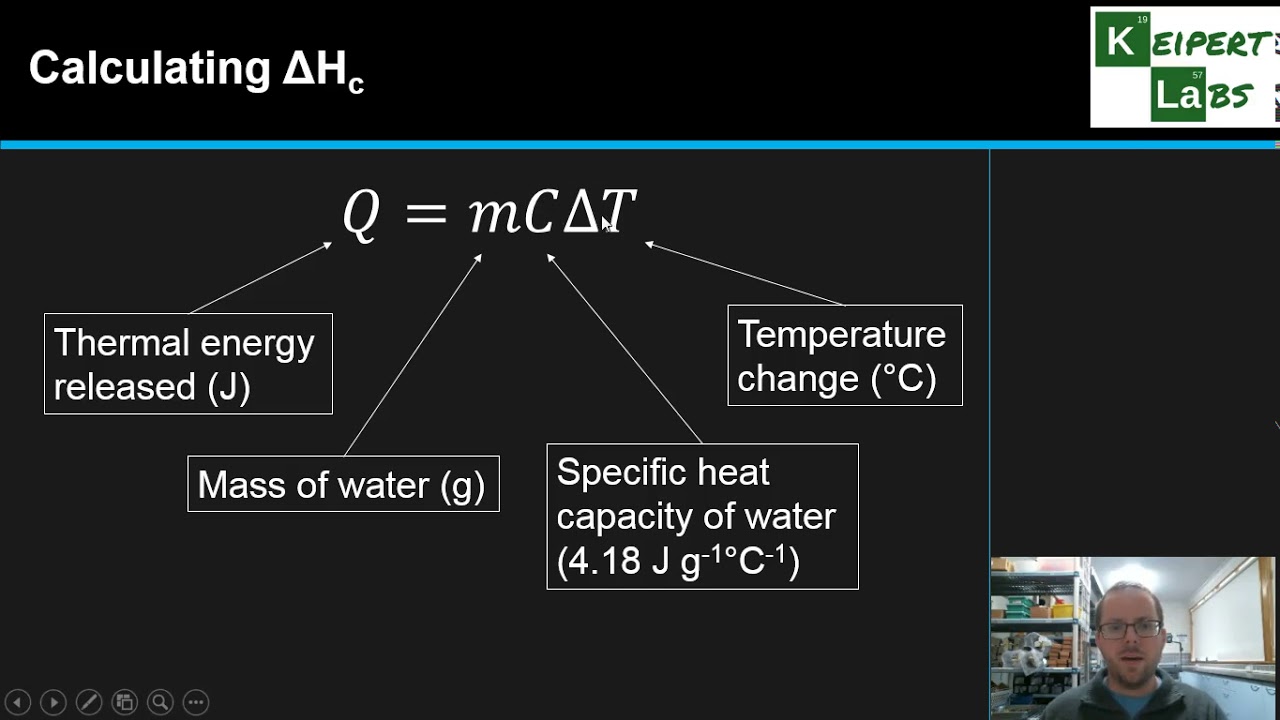
9252 we can write ΔrH Tp ξ iνiHi Tp ξ i νiCp i ΔrCp where ΔrCp is the molar reaction heat capacity at constant pressure equal to the rate at which the heat capacity Cp changes with ξ at constant T and p. C n QΔT. 1 q is the total amount of heat involved 2 ΔH vap is the symbol. Molar heat values can be looked up in reference books. Copy Sheet of paper on top of another sheet. By definition the heat of combustion enthalpy of combustion ΔHc is minus the enthalpy change for the combustion reaction ie -ΔH.
It is sometimes called heat of combustion For example the enthalpy of combustion of ethanol 13668 kJmol is the.
Standard enthalpy of combustion is the enthalpy change when 1 mole of a substance burns combines vigorously with oxygen under standard state conditions. Where Q is heat and ΔT is the change in temperature. 84 rows For a fuel of composition C c H h O o N n the higher heat of combustion is 418 kJmol c 03 h 05 o usually to a good approximation 3 though it can be significantly off if o n c for instance in the case of nitroglycerine C 3 H 5 N 3 O 9 this formula would predict a heat of combustion of 0. 9252 we can write ΔrH Tp ξ iνiHi Tp ξ i νiCp i ΔrCp where ΔrCp is the molar reaction heat capacity at constant pressure equal to the rate at which the heat capacity Cp changes with ξ at constant T and p. When an alkanol undergoes complete combustion in excess oxygen gas the products of the reaction are carbon dioxide CO 2g and water H 2 O g which will condense to H 2 O l at room. Standard enthalpy of combustion lefttextΔH_Ctextright is the enthalpy change when 1 mole of a substance burns combines vigorously with oxygen under standard state conditions.
 Source: wikihow.com
Source: wikihow.com
Wikipedia has two different pages for enthalpy of combustion and heat of combustion. It is sometimes called heat of combustion For example the enthalpy of combustion of ethanol 13668 kJmol is the. It is given the symbol ΔH c. Molar Heat of Combustion molar enthalpy of combustion of a substance is the heat liberated when 1 mole of the substance undergoes complete combustion with oxygen at constant pressure. It is sometimes called heat of combustion For example the enthalpy of combustion of ethanol 13668 kJmol is the amount of heat produced when one mole of ethanol undergoes complete combustion at 25 C.
 Source: pinterest.com
Source: pinterest.com
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. The term heat of combustion is sometimes taken to be minus deltaH and its sometimes taken to be the same as enthalpy of combustion. The heat of displacement is the heat change when one mole of a metal is displaced from its salt solution by a more electropositive metal. Copy Sheet of paper on top of another sheet. C n QΔT.
 Source: opentextbc.ca
Source: opentextbc.ca
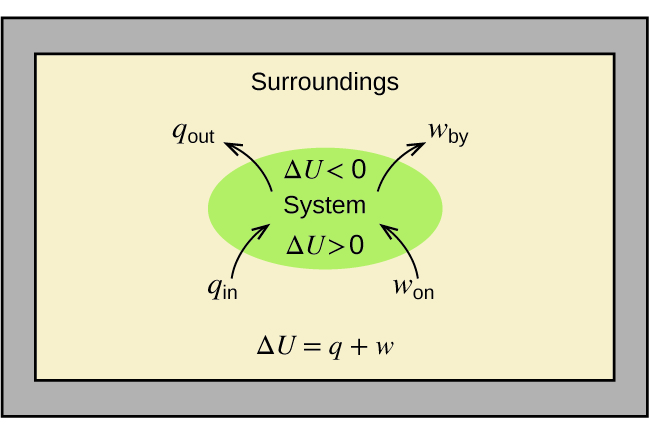
The change in energy ΔU equals the sum of heat produced and work done. Molar heat capacity or molar specific heat capacity is the amount of heat energy required to raise the temperature of 1 mole of a substance. 84 rows For a fuel of composition C c H h O o N n the higher heat of combustion is 418 kJmol c 03 h 05 o usually to a good approximation 3 though it can be significantly off if o n c for instance in the case of nitroglycerine C 3 H 5 N 3 O 9 this formula would predict a heat of combustion of 0. Copy Sheet of paper on top of another sheet. C 4 H 10 O.
 Source: youtube.com
Source: youtube.com
Where Q is heat and ΔT is the change in temperature. Wikipedia has two different pages for enthalpy of combustion and heat of combustion. It is sometimes called heat of combustion For example the enthalpy of combustion of ethanol 13668 kJmol is the amount of heat produced when one mole of ethanol undergoes. Consider the reaction of dissolving a piece of copper in concentrated nitric acid which generates a gas. Their definition is the same and they use the same symbol Δ H c It says that enthalpies of combustion are always negative as these reactions are exothermic.
 Source: ausetute.com.au
Source: ausetute.com.au
Q ΔH vap massmolar mass The meanings are as follows. The heat of displacement is the heat change when one mole of a metal is displaced from its salt solution by a more electropositive metal. Copy Sheet of paper on top of another sheet. Standard enthalpy of combustion is the enthalpy change when 1 mole of a substance burns combines vigorously with oxygen under standard state conditions. Consider the reaction of dissolving a piece of copper in concentrated nitric acid which generates a gas.
 Source: youtube.com
Source: youtube.com
The molar enthalpy of combustion is defined as the amount of heat energy released by the complete combustion of one mole of a substance. Standard enthalpy of combustion is the enthalpy change when 1 mole of a substance burns combines vigorously with oxygen under standard state conditions. 85 rows The heat of combustion of ethanol ΔH c C 2 H 6 O l 239351 6142915. Copy Sheet of paper on top of another sheet. It is sometimes called heat of combustion For example the enthalpy of combustion of ethanol 13668 kJmol is the amount of heat produced when one mole of ethanol undergoes complete combustion at 25 C.
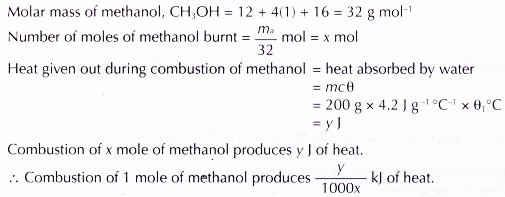 Source: aplustopper.com
Source: aplustopper.com
Molar Heat of Combustion molar enthalpy of combustion of a substance is the heat liberated when 1 mole of the substance undergoes complete combustion with oxygen at constant pressure. It is sometimes called heat of combustion For example the enthalpy of combustion of ethanol 13668 kJmol is the amount of heat produced when one mole of ethanol undergoes. Where Q is heat and ΔT is the change in temperature. B The heat of displacement of copper by zinc is -210 kJ mol-1. The enthalpy of combustion of ethene may be represented by the equation.
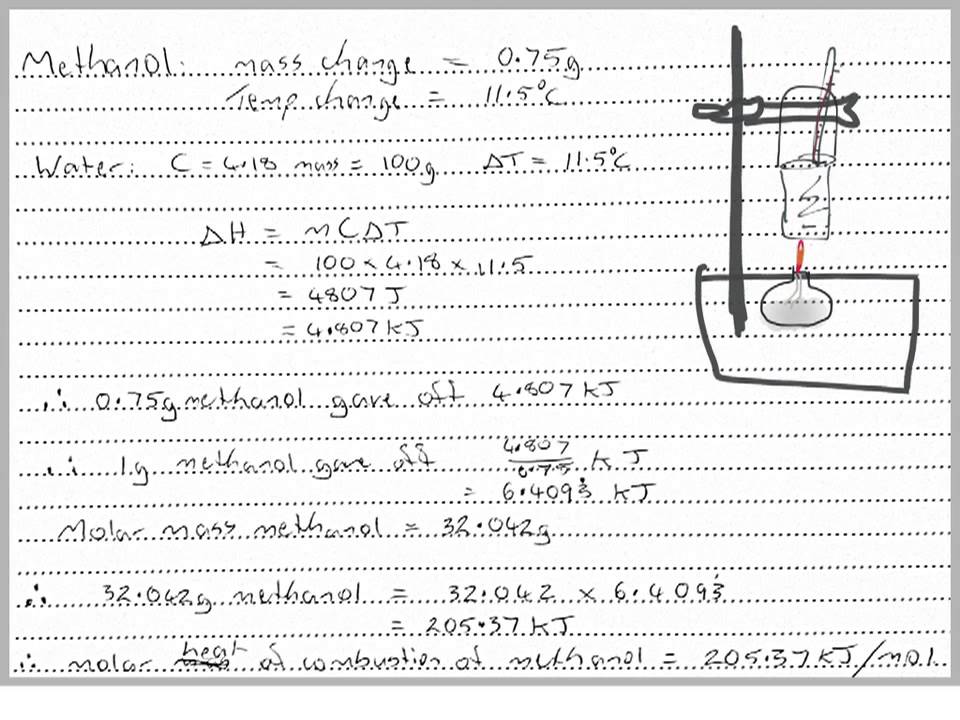 Source: youtube.com
Source: youtube.com
Consider the reaction of dissolving a piece of copper in concentrated nitric acid which generates a gas. When an alkanol undergoes complete combustion in excess oxygen gas the products of the reaction are carbon dioxide CO 2g and water H 2 O g which will condense to H 2 O l at room. It is sometimes called heat of combustion For example the enthalpy of combustion of ethanol 13668 kJmol is the amount of heat produced when one mole of ethanol undergoes complete combustion at 25 C. It is sometimes called heat of combustion For example the enthalpy of combustion of ethanol 13668 kJmol is the amount of heat produced when one mole of ethanol undergoes. Wikipedia has two different pages for enthalpy of combustion and heat of combustion.
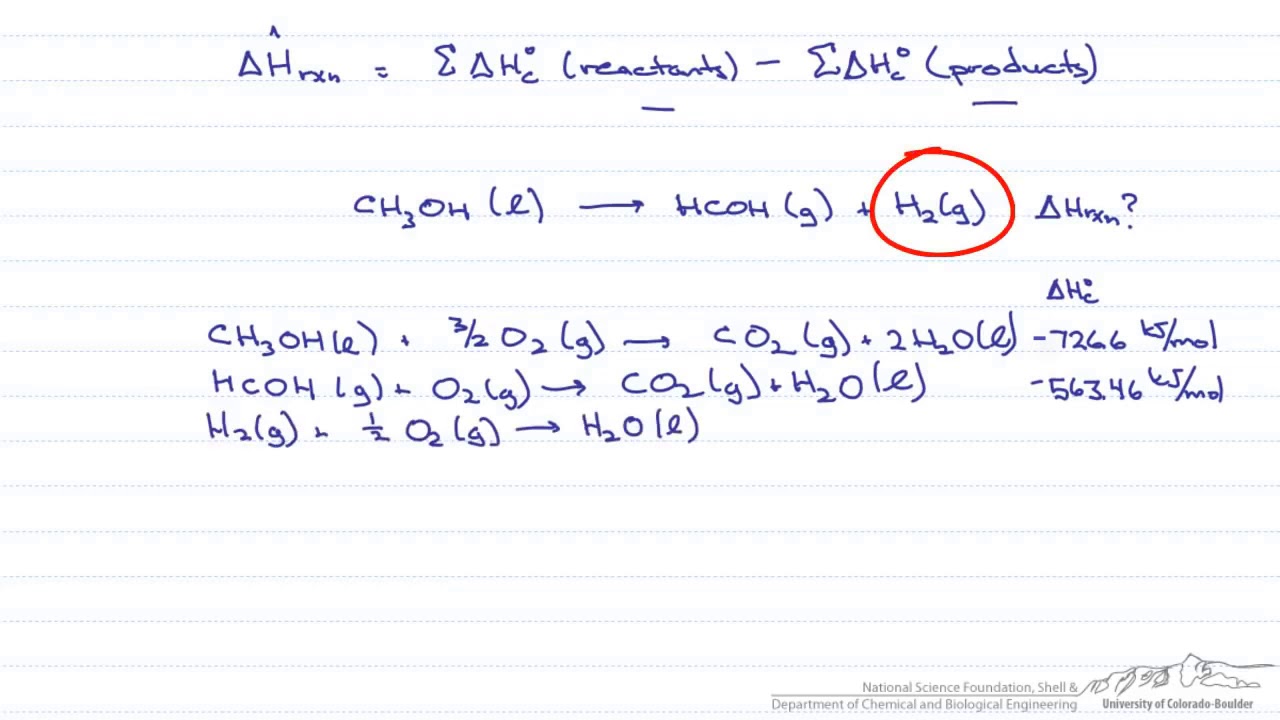 Source: youtube.com
Source: youtube.com
Click hereto get an answer to your question The molar heat of combustion of C2H2 carbon and hydrogen are - 1300 kJ - 3935 kJ and - 2826 kJ respectively. Molar Heat of Combustion molar enthalpy of combustion of a substance is the heat liberated when 1 mole of the substance undergoes complete combustion with oxygen at constant pressure. The change in energy ΔU equals the sum of heat produced and work done. The value corresponds to an exothermic reaction a negative change in. The thermochemical reaction for the displacement reaction of copper by zinc can be represented as follows.
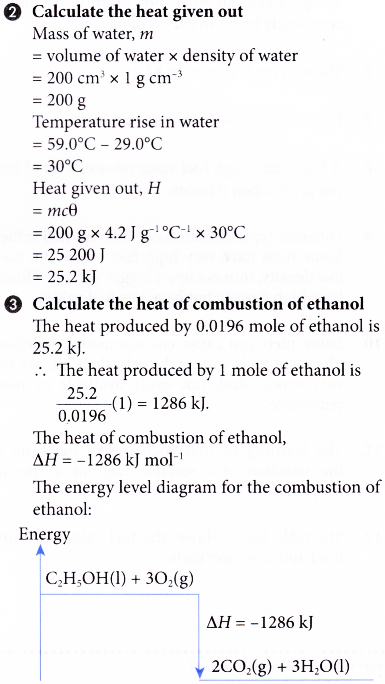 Source: aplustopper.com
Source: aplustopper.com
Molar enthalpy can also be defined as the potential energy change per one mole of a substance and it is represented by the symbol 훥퐻푥 where x signifies the type of physical or chemical change i. This reactions chemical equation is as follows. Using the relations ΔrH iνiHi from Eq. The molar enthalpy of combustion is defined as the amount of heat energy released by the complete combustion of one mole of a substance. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators.
 Source: youtube.com
Source: youtube.com
The molar enthalpy of combustion is defined as the amount of heat energy released by the complete combustion of one mole of a substance. Q ΔH vap massmolar mass The meanings are as follows. Wikipedia has two different pages for enthalpy of combustion and heat of combustion. This reactions chemical equation is as follows. 85 rows The heat of combustion of ethanol ΔH c C 2 H 6 O l 239351 6142915.
 Source: nagwa.com
Source: nagwa.com
The pressure-volume work performed by an expanding gas is known as pressure-volume work or just PV work. The heat of displacement is the heat change when one mole of a metal is displaced from its salt solution by a more electropositive metal. C n QΔT. C 2 H 4 g 2O 2 g 2CO 2 g 2H 2 O l ΔH -1411 kJ. The molar enthalpy of combustion is defined as the amount of heat energy released by the complete combustion of one mole of a substance.
 Source: youtube.com
Source: youtube.com
Standard enthalpy of combustion lefttextΔH_Ctextright is the enthalpy change when 1 mole of a substance burns combines vigorously with oxygen under standard state conditions. The molar heat of combustion left He right is the heat released when one mole of a substance is completely burned. When an alkanol undergoes complete combustion in excess oxygen gas the products of the reaction are carbon dioxide CO 2g and water H 2 O g which will condense to H 2 O l at room. The heat of combustion tables list positive values for all substances. Cus 4HNO 3 aq.
 Source: youtube.com
Source: youtube.com
Copy Sheet of paper on top of another sheet. The energy released when one mole of a substance is burned in excess oxygen or air under standard conditions. Where Q is heat and ΔT is the change in temperature. Molar heat capacity or molar specific heat capacity is the amount of heat energy required to raise the temperature of 1 mole of a substance. It is sometimes called heat of combustion For example the enthalpy of combustion of ethanol 13668 kJmol is the amount of heat produced when one mole of ethanol undergoes.
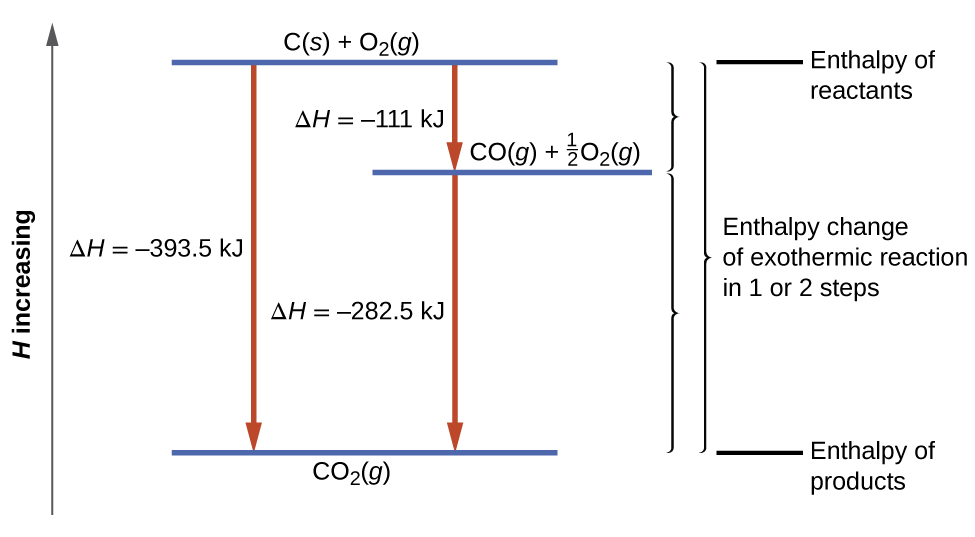 Source: opentextbc.ca
Source: opentextbc.ca
Consider the reaction of dissolving a piece of copper in concentrated nitric acid which generates a gas. The molar heat of combustion of the alkane molar enthalpy of combustion of the alkane is the amount of heat energy released when 1 mole of the alkane combusts in excess oxygen gas. The heat of combustion tables list positive values for all substances. The enthalpy of combustion of ethene may be represented by the equation. Cus 4HNO 3 aq.
 Source: easychem.com.au
Source: easychem.com.au
The energy released when one mole of a substance is burned in excess oxygen or air under standard conditions. The molar heat of vaporization equation looks like this. Q ΔH vap massmolar mass The meanings are as follows. Consider the reaction of dissolving a piece of copper in concentrated nitric acid which generates a gas. Standard enthalpy of combustion Δ H C Δ H C is the enthalpy change when 1 mole of a substance burns combines vigorously with oxygen under standard state conditions.
 Source: study.com
Source: study.com
Q ΔH vap massmolar mass The meanings are as follows. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. To get the heat of vaporization you simply divide the molar heat by 18015 gmol. The value corresponds to an exothermic reaction a negative change in. Molar heat values can be looked up in reference books.
 Source: aplustopper.com
Source: aplustopper.com
Cus 4HNO 3 aq. 85 rows The heat of combustion of ethanol ΔH c C 2 H 6 O l 239351 6142915. C 4 H 10 O. 9252 we can write ΔrH Tp ξ iνiHi Tp ξ i νiCp i ΔrCp where ΔrCp is the molar reaction heat capacity at constant pressure equal to the rate at which the heat capacity Cp changes with ξ at constant T and p. 1 q is the total amount of heat involved 2 ΔH vap is the symbol.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title molar heat of combustion symbol by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






