Your Lewis symbol for o2 images are ready. Lewis symbol for o2 are a topic that is being searched for and liked by netizens now. You can Download the Lewis symbol for o2 files here. Download all free photos.
If you’re looking for lewis symbol for o2 pictures information connected with to the lewis symbol for o2 interest, you have come to the ideal site. Our website frequently provides you with hints for downloading the highest quality video and image content, please kindly hunt and find more informative video content and images that fit your interests.
Lewis Symbol For O2. Hydrogen Oxygen Chlorine Chloride ion. Each has a total of 6 valence atoms making a sum total of 12. It also is a good example of a molecule with a double bond. There are a total of 14 valence electrons in O22-.
 H2o2 Lewis Structure Hydrogen Peroxide Hydrogen Atom Hydrogen Peroxide Molecules From pinterest.com
H2o2 Lewis Structure Hydrogen Peroxide Hydrogen Atom Hydrogen Peroxide Molecules From pinterest.com
O22- Lewis Structure Viewing Notes. Due to oxygens high electronegativity affinity for electrons the pure element is nearly exclusively found in either this. An einer Bindung sind laut der Lewis Schreibweise nur die Valenzelektronen beteiligt. A step-by-step explanation of how to draw the O2- Lewis Dot StructureFor the O 2- structure use the periodic table to find the total number of valence elect. There are a total of 14 valence electrons in O22-. Um aber die Schreibweise noch weiter zu vereinfachen werden zwei beieinander liegende Elektronen ein Elektronenpaar zu einem Strich verbunden.
Nachdem 4 Valenzelektronen um das Elementsymbol gruppiert sind beginnt man wieder.
How to draw the Lewis Structure of Oxygen Gas - with explanationCheck me out. Want this question answered. Option A is wrong because a single bond. How many electrons are in the Lewis dot diagram of an O2 ion. Each has a total of 6 valence atoms making a sum total of 12. There are 12 valence electrons available for the Lewis structure for O Video.
 Source: pinterest.com
Source: pinterest.com
What are the rules for drawing Lewis dot structures. A MgS b Al 2 O 3 c GaCl 3 d K 2 O e Li 3 N f KF. What are the rules for drawing Lewis dot structures. How can one draw a Lewis structure and use it to understand how atoms bond together to make molecules. Asked Sep 1 2019 in Chemistry by jgrier1.
 Source: learnwithdrscott.com
Source: learnwithdrscott.com
Option A is wrong because a single bond. Basierend auf der Oktettregel und den unterschiedlichen Bindungsarten in der Chemie werden in der Lewis Schreibweise auch die Bindungen zwischen den Atomen abgebildet. The nuclei contain the protons and neutrons which are the solid parts of the molecule. Drawing the Lewis Structure for O 2. Interestingly the dots and lines represent electrons which are not solid.
 Source: pinterest.com
Source: pinterest.com
2 O24 Fe2 Fe2O3 C7H1610 O27 CO28 H2O FeBr3H2SO4Fe2SO43HBr 2 Fe3 Cl22 FeCl3. Punkteschreibweise links übliche Strichschreibweise rechts. Asked Sep 1 2019 in Chemistry by jgrier1. O O Its a very simple structure but how does one interpret this Lewis structure. What are the rules for drawing Lewis dot structures.
 Source: pinterest.com
Source: pinterest.com
The nuclei contain the protons and neutrons which are the solid parts of the molecule. There are 12 valence electrons available for the Lewis structure for O2. Each dot represents one electron. Answered Sep 1 2019 by Aevinece. Draw the Lewis dot structures of the following.
 Source: pinterest.com
Source: pinterest.com
For example oxygen has 6 valence electrons so we write the symbol O for oxygen and surround it with 6 dots. Write the Lewis symbols of the ions in each of the following ionic compounds and the Lewis symbols of the atom from which they are formed. Draw the Lewis dot structures of the following. The Lewis Dot Structure for O2 or dioxygen is as follows. Oxygen O2 is a commonly tested Lewis structure due to its importance on Earth.
 Source: pinterest.com
Source: pinterest.com
The nuclei contain the protons and neutrons which are the solid parts of the molecule. Mit der LEWIS-Formel würde man dies als HH amerikanische Schreibweise darstellen. The two oxygen atom can both achieve a stable structure by sharing two pairs of electrons. OMGThis is soo helpful. Draw the Lewis dot structures of the following.
 Source: pinterest.com
Source: pinterest.com
The two oxygen atom can both achieve a stable structure by sharing two pairs of electrons. Write the name of the following ionic compounds. 2 O24 Fe2 Fe2O3 C7H1610 O27 CO28 H2O FeBr3H2SO4Fe2SO43HBr 2 Fe3 Cl22 FeCl3. So according to the lewis dot structure of OF2 oxygen is the central atom and it has 2 bonded pair electrons and 2 lone pairs of electrons. Die Lewis-Schreibweise oft auch als Lewis-Formel bezeichnet sieht für das Kohlenstoffatom folgendermaßen aus.
 Source: makethebrainhappy.com
Source: makethebrainhappy.com
O22- Lewis Structure Viewing Notes. Option A is wrong because a single bond. Nachdem 4 Valenzelektronen um das Elementsymbol gruppiert sind beginnt man wieder. There are 12 valence electrons available for the Lewis structure for O Video. OMGThis is soo helpful.
 Source: pinterest.com
Source: pinterest.com
With O22- be sure to add two additional valence electrons to your total because of the negative two in the chemical formula. There are 12 valence electrons available for the Lewis structure for O2. There are a total of 14 valence electrons in O22-. Man schreibt also das Symbol des Elements aus dem Periodensystem und einen Punkt für jeweils ein Valenzelektron. Write the formula of each compound using the chemical symbols of each.
 Source: pinterest.com
Source: pinterest.com
What are the rules for drawing Lewis dot structures. It also is a good example of a molecule with a double bond. Sind zwei verschiedene Elemente miteinander verbunden handelt. Man schreibt also das Symbol des Elements aus dem Periodensystem und einen Punkt für jeweils ein Valenzelektron. Due to oxygens high electronegativity affinity for electrons the pure element is nearly exclusively found in either this.

Each dot represents one electron. Sodium and fluorine sodium and iodine sodium and oxygen sodium and sulfur barium and oxygen chromium. Drawing the Lewis Structure for O 2. How many electrons are in the Lewis dot diagram of an O2 ion. Option A is wrong because a single bond.
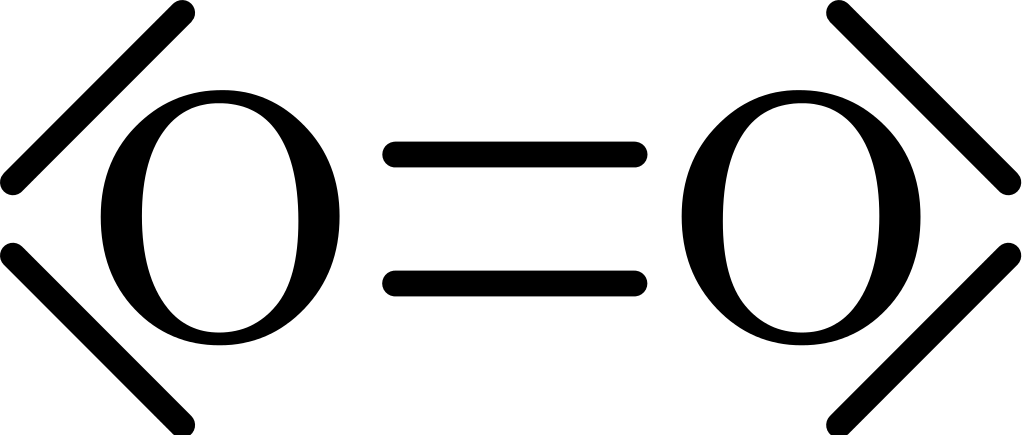 Source: commons.wikimedia.org
Source: commons.wikimedia.org
Each has a total of 6 valence atoms making a sum total of 12. The double bond they share is denoted by the double lines joining the two atoms. Draw the Lewis dot structures of the following. Nachdem 4 Valenzelektronen um das Elementsymbol gruppiert sind beginnt man wieder. Due to this reason it is essential to study its Lewis structure.
 Source: pinterest.com
Source: pinterest.com
Want this question answered. Oxygen O2 is a commonly tested Lewis structure due to its importance on Earth. There are 12 valence electrons available for the Lewis structure for O Video. Drawing the Lewis Structure for O 2. Each has a total of 6 valence atoms making a sum total of 12.
 Source: commons.wikimedia.org
Source: commons.wikimedia.org
Sodium and fluorine sodium and iodine sodium and oxygen sodium and sulfur barium and oxygen chromium. OMGThis is soo helpful. Each has a total of 6 valence atoms making a sum total of 12. Write the chemical formula for the ionic compound of the elements. The nuclei contain the protons and neutrons which are the solid parts of the molecule.
 Source: pinterest.com
Source: pinterest.com
Answered Sep 1 2019 by acterr01. Want this question answered. How to draw the Lewis Structure of Oxygen Gas - with explanationCheck me out. Each dot represents one electron. O2 is a chemical element within the 16th group of the periodic table called chalcogens.
 Source: pinterest.com
Source: pinterest.com
The existence of a strong shared covalent double bond between the two oxygen molecules within a. Each has a total of 6 valence atoms making a sum total of 12. When using the indirect method to prepare the operating section of a statement of cash flows which of the following is deducted from. Mit der LEWIS-Formel würde man dies als HH amerikanische Schreibweise darstellen. It also is a good example of a molecule with a double bond.
 Source: in.pinterest.com
Source: in.pinterest.com
With O22- be sure to add two additional valence electrons to your total because of the negative two in the chemical formula. Die Lewis-Schreibweise oft auch als Lewis-Formel bezeichnet sieht für das Kohlenstoffatom folgendermaßen aus. Interestingly the dots and lines represent electrons which are not solid. The double bond they share is denoted by the double lines joining the two atoms. Due to this reason it is essential to study its Lewis structure.
 Source: pinterest.com
Source: pinterest.com
O O Its a very simple structure but how does one interpret this Lewis structure. The two oxygen atom can both achieve a stable structure by sharing two pairs of electrons. Each dot represents one electron. A step-by-step explanation of how to draw the O2- Lewis Dot StructureFor the O 2- structure use the periodic table to find the total number of valence elect. OMGThis is soo helpful.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title lewis symbol for o2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






