Your Lewis symbol for chlorine images are available in this site. Lewis symbol for chlorine are a topic that is being searched for and liked by netizens today. You can Find and Download the Lewis symbol for chlorine files here. Download all royalty-free photos.
If you’re looking for lewis symbol for chlorine images information linked to the lewis symbol for chlorine topic, you have visit the ideal blog. Our site frequently gives you suggestions for seeing the highest quality video and image content, please kindly search and locate more informative video articles and graphics that match your interests.
Lewis Symbol For Chlorine. Hydrogen Oxygen Chlorine Chloride ion. Chlorine atom in ClO2- lewis structure expanded the octet because it has d-orbitals in the third principal energy level hence it has extra orbitald-orbital for additional electron needed for bonding. A 42 B 41 C 61 D 05 E 25 FREE Expert Solution Hey there. Left Ne right 3s1.
 Ch2cl2 Lewis Structure Dichloromethane Molecules Hydrogen Atom Lewis From pinterest.com
Ch2cl2 Lewis Structure Dichloromethane Molecules Hydrogen Atom Lewis From pinterest.com
Sulfur phosphorous and silicon are some other examples that can expand their octet and hold electrons more than 8. Im Periodensystem der Elemente steht es in der 7. The two atoms have these Lewis electron dot diagrams and electron configurations. Cl 2 is a greenish yellow gas and is a strong oxidizing agent. A 42 B 41 C 61 D 05 E 25 FREE Expert Solution Hey there. Explain how magnesium and chlorine atoms obtain the octet configuration in the formation of ionic compound.
There are _____ paired and _____ unpaired electrons in the Lewis symbol for a chlorine atom.
The Lewis structure indicates that each Cl atom has three pairs of electrons that are. Chlorine atom is present in group 7 halogen gro View the full answer Transcribed image text. The Lewis structure indicates that each Cl atom has three pairs of electrons that are. The Lewis dot structure for Cl 2 shows. To answer this we need to do the following steps. Chlor ist ein chemisches Element mit dem Symbol Cl und der Ordnungszahl 17.
 Source: pinterest.com
Source: pinterest.com
The two atoms have these Lewis electron dot diagrams and electron configurations. A chlorine atom has 17 electrons but only 7 of these are. That means in my Trojan there are three single electrons on one lone pair off electron. So carbon is group for A this is also considered Group 14. There are two single.
 Source: in.pinterest.com
Source: in.pinterest.com
Chlorine atom in ClO2- lewis structure expanded the octet because it has d-orbitals in the third principal energy level hence it has extra orbitald-orbital for additional electron needed for bonding. The Lewis Dot Structure for Cl2. Cl 2 is a greenish yellow gas and is a strong oxidizing agent. The two atoms have these Lewis electron dot diagrams and electron configurations. There are two single.
 Source: commons.wikimedia.org
Source: commons.wikimedia.org
Chlorine gas exists in diatomic form as Cl 2. We also use Lewis symbols to indicate the formation of covalent bonds which are shown in Lewis structures drawings that describe the bonding in molecules and polyatomic ionsFor example when two chlorine atoms form a chlorine molecule they share one pair of electrons. Why does the molecular geometry of ClO2- appears bent in. We write chlorine as CL2 because chlorine is a non metallic gas. So carbon is group for A this is also considered Group 14.
 Source: pinterest.com
Source: pinterest.com
Chlor ist ein chemisches Element mit dem Symbol Cl und der Ordnungszahl 17. The Lewis Dot Structure for Cl2. Calculate the total number of valence electrons. To answer this we need to do the following steps. Chlorine atom is present in group 7 halogen gro View the full answer Transcribed image text.
 Source: pinterest.com
Source: pinterest.com
Why does the molecular geometry of ClO2- appears bent in. Chlorine atom in ClO2- lewis structure expanded the octet because it has d-orbitals in the third principal energy level hence it has extra orbitald-orbital for additional electron needed for bonding. Thus we draw the Lewis structure for a sodium atom as the symbol Na with a single dot. Therefore the best Lewis dot structure for chlorine Cl is structure c. What is the Lewis symbol for potassium and chloride ions.
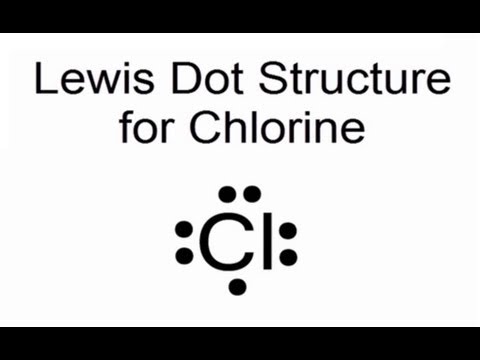 Source: howtodiscuss.com
Source: howtodiscuss.com
Thus we draw the Lewis structure for a sodium atom as the symbol Na with a single dot. State the octet rule. The Lewis structure indicates that each Cl atom has three pairs of electrons that are. Therefore the best Lewis dot structure for chlorine Cl is structure c. To answer this we need to do the following steps.
 Source: pinterest.com
Source: pinterest.com
The symbol for chlorine is Cl. So firstly we have carbon. Lewis electron-dot symbols work well for the representative elements. Now consider an Li atom in the presence of a Cl atom. Lewis symbols can also be used to illustrate the formation of cations from atoms as shown here for sodium and calcium.
 Source: pinterest.com
Source: pinterest.com
What are the chemical reactions in pool chlorine. So firstly we have carbon. In an electron dot diagram this symbol represents the nucleus and the ten electrons in the first two energy levels. Lewis electron-dot symbols work well for the representative elements. By using the Lewis dot symbol describe the formation of magnesium chloride compound 9 marks 2.
 Source: pinterest.com
Source: pinterest.com
The chemical symbol for Chlorine is Cl. In an electron dot diagram this symbol represents the nucleus and the ten electrons in the first two energy levels. Sulfur phosphorous and silicon are some other examples that can expand their octet and hold electrons more than 8. Want this question answered. That means in my Trojan there are three single electrons on one lone pair off electron.
 Source: pinterest.com
Source: pinterest.com
Chemical reactions in swimming pool chlorine chemistry Part of chlorine reactions in water is that as you use HOCL the. Who were looking at the periodic table of elements and were looking to write out the group name and the valence electron structure of each of the following elements. Hauptgruppe und gehört damit zusammen mit Fluor Brom Iod Astat und Tenness zur 17. Chemical reactions in swimming pool chlorine chemistry Part of chlorine reactions in water is that as you use HOCL the. Lewis electron-dot symbols work well for the representative elements.
 Source: pinterest.com
Source: pinterest.com
Were being asked to determine the paired and unpaired electrons in the Lewis formula for a chlorine atom. Why does the molecular geometry of ClO2- appears bent in. What is the Lewis symbol for potassium and chloride ions. In an electron dot diagram this symbol represents the nucleus and the ten electrons in the first two energy levels. Hauptgruppe und gehört damit zusammen mit Fluor Brom Iod Astat und Tenness zur 17.
 Source: pinterest.com
Source: pinterest.com
Im Periodensystem der Elemente steht es in der 7. Write the Lewis dot symbol for magnesium and chlorine atoms. Want this question answered. Lewis electron-dot symbols work well for the representative elements. The Lewis structure indicates that each Cl atom has three pairs of electrons that are.
 Source: pinterest.com
Source: pinterest.com
Hauptgruppe und gehört damit zusammen mit Fluor Brom Iod Astat und Tenness zur 17. Demonstrates the use of Lewis symbols to show the transfer of electrons during the formation of ionic compounds. Left Ne right 3s1. Now consider an Li atom in the presence of a Cl atom. Also as chlorine is a gas it occurs in diatomic form as cl2 such as hydrogenh2.
 Source: pinterest.com
Source: pinterest.com
Left Ne right 3s1. The Lewis dot structure for Cl 2 shows. IUPAC-Gruppe den HalogenenElementares Chlor liegt unter Normalbedingungen in Form des zweiatomigen Moleküls Cl 2 gasförmig vor. Request Answer. Want this question answered.
 Source: pinterest.com
Source: pinterest.com
Hydrogen Oxygen Chlorine Chloride ion. The Lewis Dot Structure for Cl2. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. State the octet rule. Louis symbol is a chemical symbol for him out of which is surrounded by one or more thoughts that we present the number off Valence electrons for night Trojan the Louis symbol will be 1234 Why you and six.
 Source: pinterest.com
Source: pinterest.com
We write chlorine as CL2 because chlorine is a non metallic gas. Hauptgruppe und gehört damit zusammen mit Fluor Brom Iod Astat und Tenness zur 17. Were being asked to determine the paired and unpaired electrons in the Lewis formula for a chlorine atom. A Lewis symbolis a symbol in which the electrons in the valence shell of an atom or simple ion are represented by dots placed around the letter symbol of the element. We write chlorine as CL2 because chlorine is a non metallic gas.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
A Lewis symbolis a symbol in which the electrons in the valence shell of an atom or simple ion are represented by dots placed around the letter symbol of the element. Left Ne right 3s1. Explain how magnesium and chlorine atoms obtain the octet configuration in the formation of ionic compound. A Lewis symbolis a symbol in which the electrons in the valence shell of an atom or simple ion are represented by dots placed around the letter symbol of the element. The symbol for chlorine is Cl.
 Source: pinterest.com
Source: pinterest.com
Chlor ist ein chemisches Element mit dem Symbol Cl und der Ordnungszahl 17. Im Periodensystem der Elemente steht es in der 7. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Cl 2 is a greenish yellow gas and is a strong oxidizing agent. A 42 B 41 C 61 D 05 E 25 FREE Expert Solution Hey there.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title lewis symbol for chlorine by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






