Your Lewis symbol for carbon images are available. Lewis symbol for carbon are a topic that is being searched for and liked by netizens today. You can Find and Download the Lewis symbol for carbon files here. Download all royalty-free images.
If you’re looking for lewis symbol for carbon images information linked to the lewis symbol for carbon keyword, you have pay a visit to the right blog. Our site frequently provides you with hints for seeing the highest quality video and picture content, please kindly search and locate more informative video content and images that fit your interests.
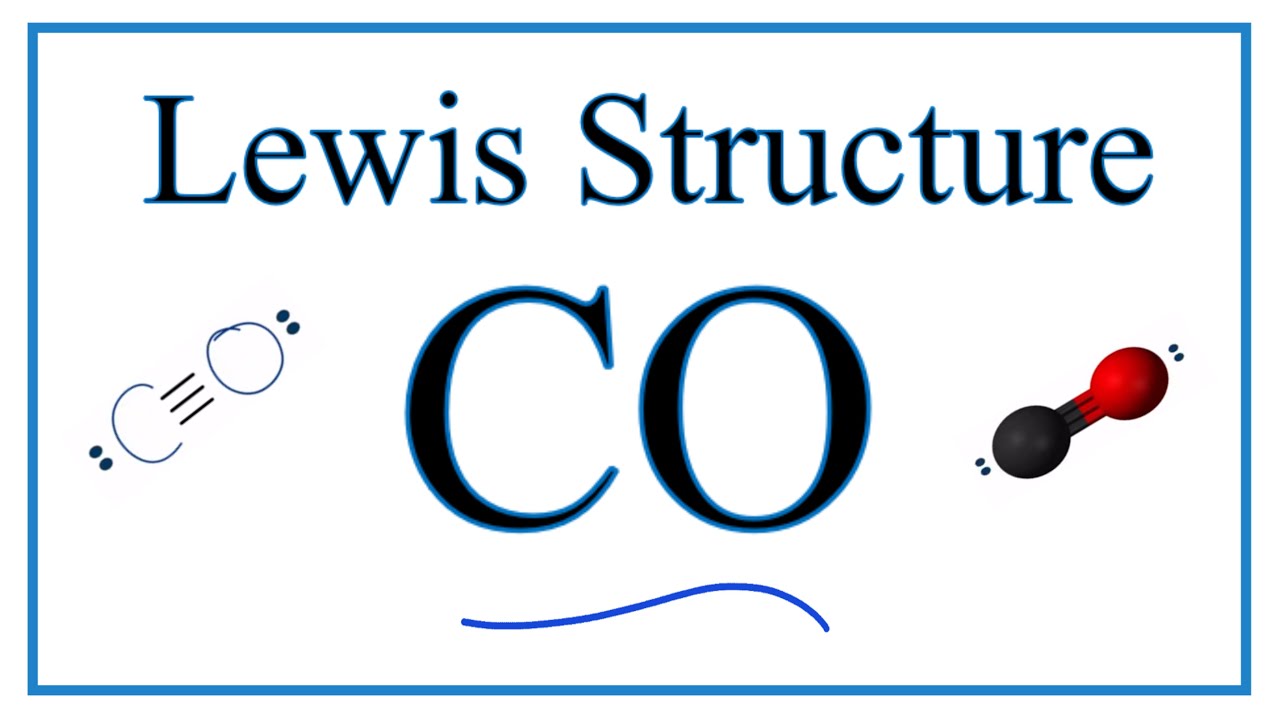
Lewis Symbol For Carbon. The Lewis structure for CO has 10 valence electrons. Because hydrogen only needs two electrons to fill its valence shell it is an exception to the octet rule. The number of bonds which carbon usually forms in order to complete its valence shell and obey the octet rule is ___ a. The number of bonds which.

Oxygen contains 6 valence electrons which form 2 lone pairs. Lewis dot diagram of Carbon This video shows how to use the periodic table to draw Lewis structures and figure out how many valence electrons an atom has. When we draw the Lewis Structure for the Carbon youll put four dots or valance electrons around the element symbol C. The number of covalent bonds which carbon usually forms in order to complete its valence shell and obey the octet rule is ___. The Lewis symbol for the carbon atom shows __ valence electrons. Clear discussion of Carbon valence electrons lewis dot.
Because hydrogen only needs two electrons to fill its valence shell it is an exception to the octet rule.
Also since nitrogen must gain three electrons to achieve a noble-gas configuration it will usually form three covalent bonds. In C O 2 oxygen belongs to group 16 of the Periodic Table and carbon belongs to group 14 of the Periodic Table. The maximum number of valence electron dots in the Lewis electron dot diagram is begin align8end align. Notice the correspondence to each elements group number. Because hydrogen only needs two electrons to fill its valence shell it is an exception to the octet rule. Hence oxygen has 6 valence electrons and carbon has 4.
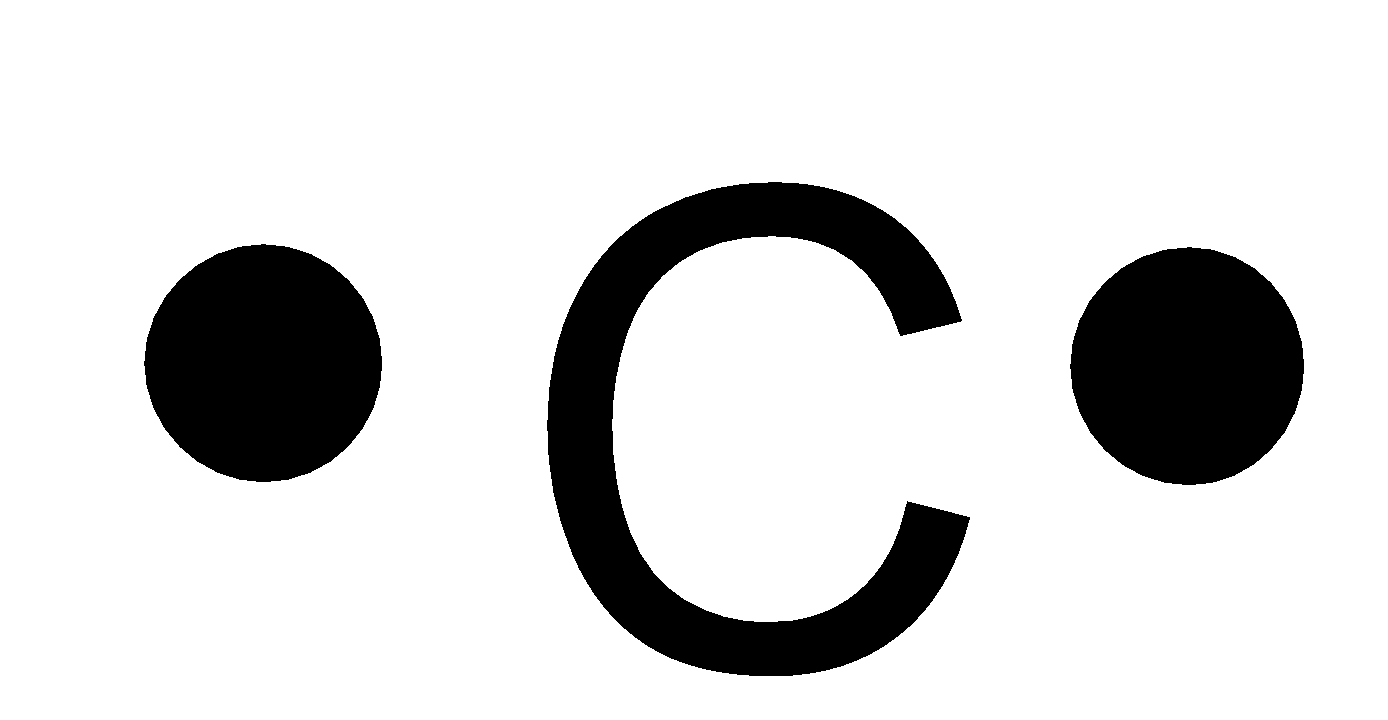 Source: vedantu.com
Source: vedantu.com
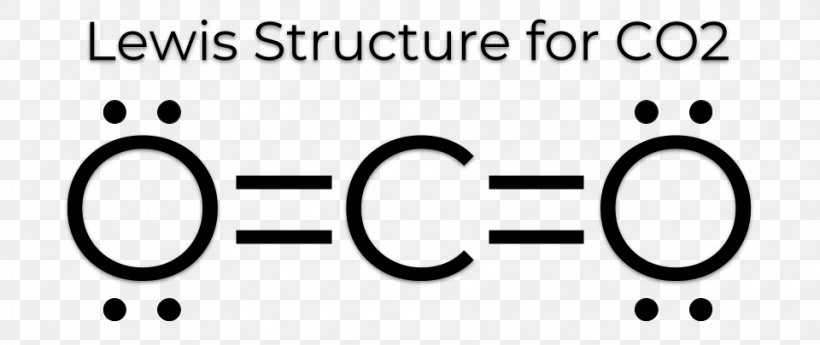
In C O 2 oxygen belongs to group 16 of the Periodic Table and carbon belongs to group 14 of the Periodic Table. Each of the four valence electrons is represented as a dot. These four electrons can be gained by forming four covalent bonds as illustrated here for carbon in CCl 4 carbon tetrachloride and silicon in SiH 4 silane. The number of bonds which carbon usually forms in order to complete its valence shell and obey the octet rule is ___ a. CO2 Lewis structure So CO2 4 6 2 16.
 Source: studylib.net
Source: studylib.net
Each of the four valence electrons is represented as a dot. These four electrons can be gained by forming four covalent bonds as illustrated here for carbon in CCl 4 carbon tetrachloride and silicon in SiH 4 silane. What is the Lewis dot diagram for carbon. The Lewis electron dot structures of a few molecules are illustrated in this subsection. Also since nitrogen must gain three electrons to achieve a noble-gas configuration it will usually form three covalent bonds.
![]() Source: study.com
Source: study.com
Draw Lewis structures for atoms ions and simple molecules. Which element will have 5 electrons in its Lewis dot symbol. Clear discussion of Carbon valence electrons lewis dot. Read complete answer here. CO2 Lewis structure So CO2 4 6 2 16.
 Source: socratic.org
Source: socratic.org
Scientists often create models to represent either a physical or abstract system or structure. Below are Lewis Symbols for various elements. Draw Lewis structures for atoms ions and simple molecules. Carbon has four valence electrons and therefore they are drawn on the four sides of a carbon atom as represented in the figures below. The Lewis symbol for the nitrogen atom shows __ valence electrons.

Hence oxygen has 6 valence electrons and carbon has 4. How to Draw the Lewis Dot Structure for Carbon monoxide It is helpful if you. What is the maximum number of dots in a Lewis dot structure. Scientists often create models to represent either a physical or abstract system or structure. What is the Lewis dot diagram for carbon.
 Source: youtube.com
Source: youtube.com
Each of the four valence electrons is represented as a dot. Thus the Lewis Carbon structure is letter C symbol for Carbon representing the Carbon core of six protons and six neutrons and two s electrons. The Lewis structure for CO has 10 valence electrons. It features the distribution of valence electrons around elements. Which element will have 5 electrons in its Lewis dot symbol.
 Source: learnwithdrscott.com
Source: learnwithdrscott.com
It features the distribution of valence electrons around elements. For many common elements the number of dots corresponds to the elements group number. A clear explanation of valence electrons and how to draw lewis dot symbol of group IV A elements. Scientists often create models to represent either a physical or abstract system or structure. Hence oxygen has 6 valence electrons and carbon has 4.

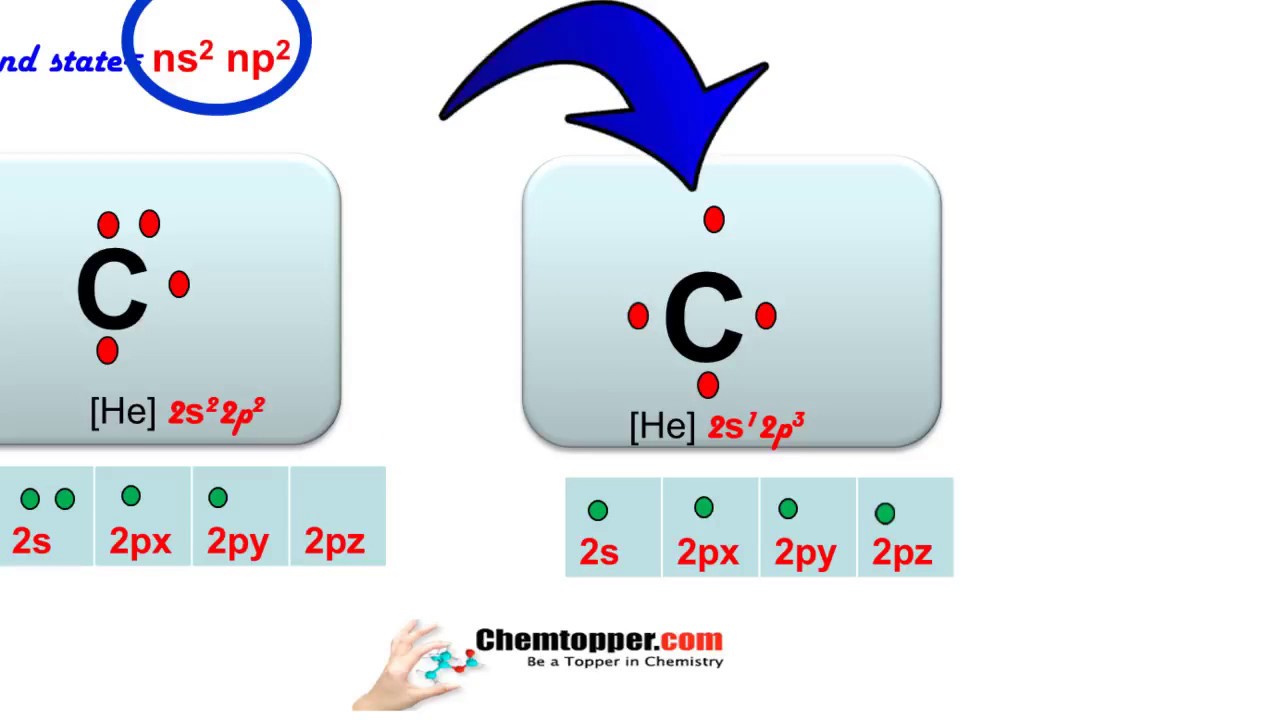
Use Lewis structures as a guide to construct three-dimensional models of small molecules. A Lewis Symbol is constructed by placing dots representing electrons in the outer energy around the symbol for the element. In the structure of Lewis the entire atom with the exception of the valence electrons is represented by the symbol of the element and the valence electrons are represented by points. Thus the Lewis Carbon structure is letter C symbol for Carbon representing the Carbon core of six protons and six neutrons and two s electrons. Each of the four valence electrons is represented as a dot.
 Source: pinterest.com
Source: pinterest.com
A clear explanation of valence electrons and how to draw lewis dot symbol of group IV A elements. Read complete answer here. Which element will have 5 electrons in its Lewis dot symbol. These four electrons can be gained by forming four covalent bonds as illustrated here for carbon in CCl 4 carbon tetrachloride and silicon in SiH 4 silane. Write the correct Lewis dot structure for O2.
 Source: chemistrylearner.com
Source: chemistrylearner.com
These four electrons can be gained by forming four covalent bonds as illustrated here for carbon in CCl 4 carbon tetrachloride and silicon in SiH 4 silane. Because hydrogen only needs two electrons to fill its valence shell it is an exception to the octet rule. Because hydrogen only needs two electrons to fill its valence shell it is an exception to the octet rule and only needs to. Notice the correspondence to each elements group number. Oxygen contains 6 valence electrons which form 2 lone pairs.
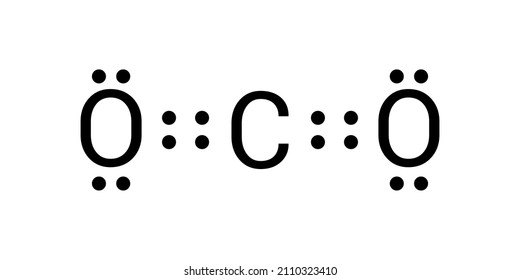 Source: shutterstock.com
Source: shutterstock.com
So total valence electrons are 16. A Lewis Symbol is constructed by placing dots representing electrons in the outer energy around the symbol for the element. Arrange the following in terms of increasing electronegativity values. How to Draw the Lewis Dot Structure for Carbon monoxide It is helpful if you. The Lewis symbol for the carbon atom has __ valence electrons.
 Source: youtube.com
Source: youtube.com
Ga P As. Because hydrogen only needs two electrons to fill its valence shell it is an exception to the octet rule and only needs to. Notice the correspondence to each elements group number. For many common elements the number of dots corresponds to the elements group number. Arrange the following in terms of increasing electronegativity values.
 Source: favpng.com
Source: favpng.com
Opo Advertisement Answer 45 5 4 hannahboo Lewis Symbol For Carbon. The number of bonds which. Determine the electron and molecular geometry of the produced molecules. Thus the Lewis Carbon structure is letter C symbol for Carbon representing the Carbon core of six protons and six neutrons and two s electrons. These four electrons can be gained by forming four covalent bonds as illustrated here for carbon in CCl 4 carbon tetrachloride and silicon in SiH 4 silane.
 Source: pngwing.com
Source: pngwing.com
Determine the electron and molecular geometry of the produced molecules. Lewis dot diagram of Carbon This video shows how to use the periodic table to draw Lewis structures and figure out how many valence electrons an atom has. Because hydrogen only needs two electrons to fill its valence shell it is an exception to the octet rule. What is the maximum number of dots in a Lewis dot structure. The Lewis electron dot structures of a few molecules are illustrated in this subsection.
 Source: youtube.com
Source: youtube.com
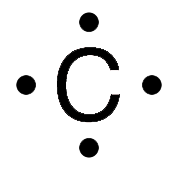
Lewis Symbols For example the Lewis symbol of carbon depicts a C surrounded by 4 valence electrons because carbon has an electron configuration of 1s22s22p2. Each of the four valence electrons is represented as a dot. In the structure of Lewis the entire atom with the exception of the valence electrons is represented by the symbol of the element and the valence electrons are represented by points. Read complete answer here. Which bond is the most polar.
 Source: youaskweanswer.net
Source: youaskweanswer.net
These four electrons can be gained by forming four covalent bonds as illustrated here for carbon in CCl 4 carbon tetrachloride and silicon in SiH 4 silane. Which of the following elements can only form one bond in a Lewis structure. For the CO Lewis structure youll need a triple bond between the Carbon and Oxygen atoms in order to satisfy the octets of each atom while still using the 10 valence electrons available for the CO molecule. Notice the correspondence to each elements group number. Which statement correctly describes the structure of the whole molecule.
 Source: youtube.com
Source: youtube.com
Ga P As. Read complete answer here. Which bond is the most polar. Also question is what is the Lewis structure for carbon dioxide. The Lewis structure for CO has 10 valence electrons.
 Source: pngwing.com
Source: pngwing.com
The Lewis symbol for carbon. Which of the following elements can only form one bond in a Lewis structure. Also question is what is the Lewis structure for carbon dioxide. The Lewis symbol for the carbon atom shows __ valence electrons. The Lewis symbol for the carbon atom has __ valence electrons.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title lewis symbol for carbon by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






