Your How to write an isotope symbol images are available. How to write an isotope symbol are a topic that is being searched for and liked by netizens today. You can Download the How to write an isotope symbol files here. Find and Download all royalty-free vectors.
If you’re looking for how to write an isotope symbol images information linked to the how to write an isotope symbol interest, you have visit the ideal blog. Our website frequently provides you with hints for refferencing the maximum quality video and image content, please kindly surf and locate more enlightening video content and graphics that match your interests.
How To Write An Isotope Symbol. Online writing service includes the research material as well but these services are for assistance purposes only. How to write an isotope symbol How is an isotope written. The atomic number A. Likewise how do you read isotope names.
 Isotopes From nglearninglab.com
Isotopes From nglearninglab.com
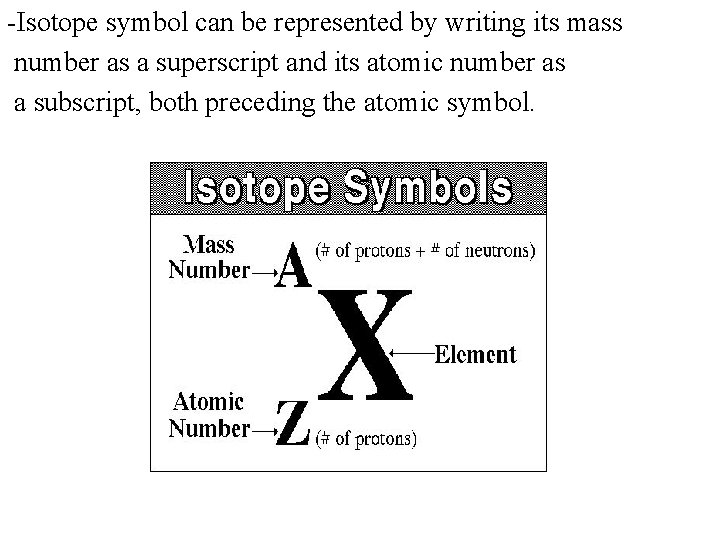
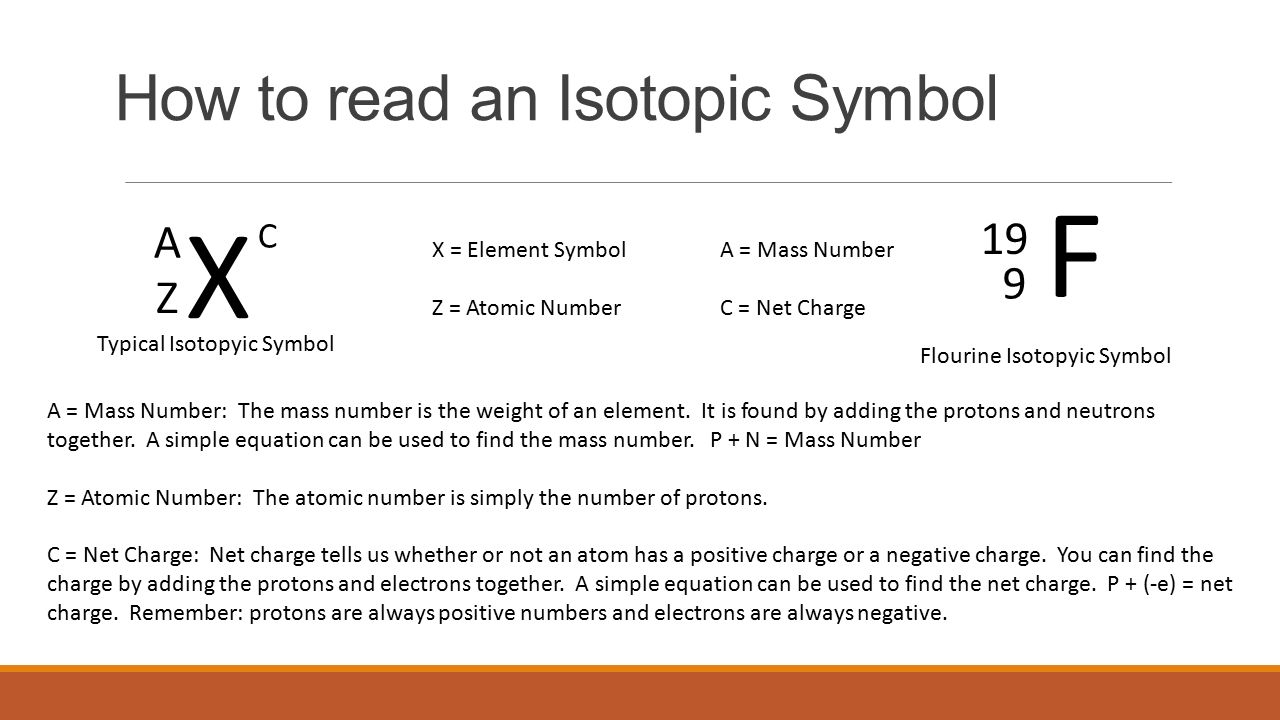
Hooks for Essay Introduction When you get the task to write an How To Write An Isotope Symbol essay professors expect you to follow the specifics of that type of essay. To write the symbol for an isotope place the atomic number as a subscript and the mass number protons plus neutrons as a superscript to the left of the atomic symbol. How many protons and neutrons are there in the nucleus of strontium-90. Write isotopic symbols of the form X-A for example C-13 for each isotopes. While its common to write nuclear symbols with the atomic massthe sum of the number of protons and neutronsas a superscript and atomic number the number of protons as a subscript theres an easier way to indicate nuclear symbols. By following the AZE notation also known as the standard notation.
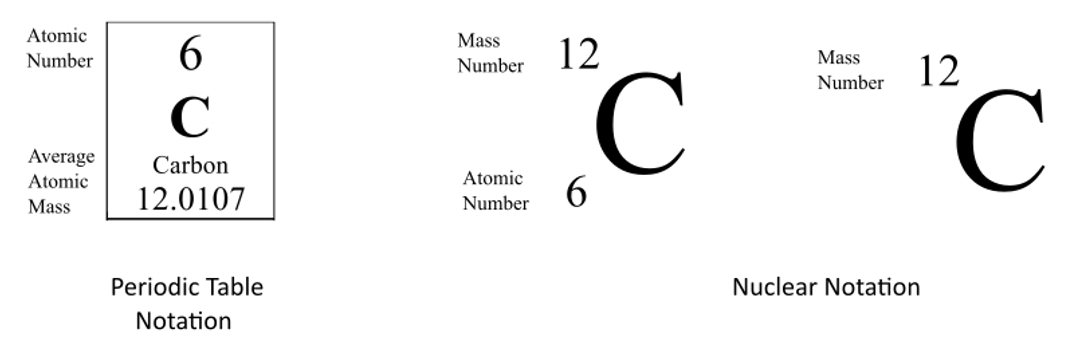
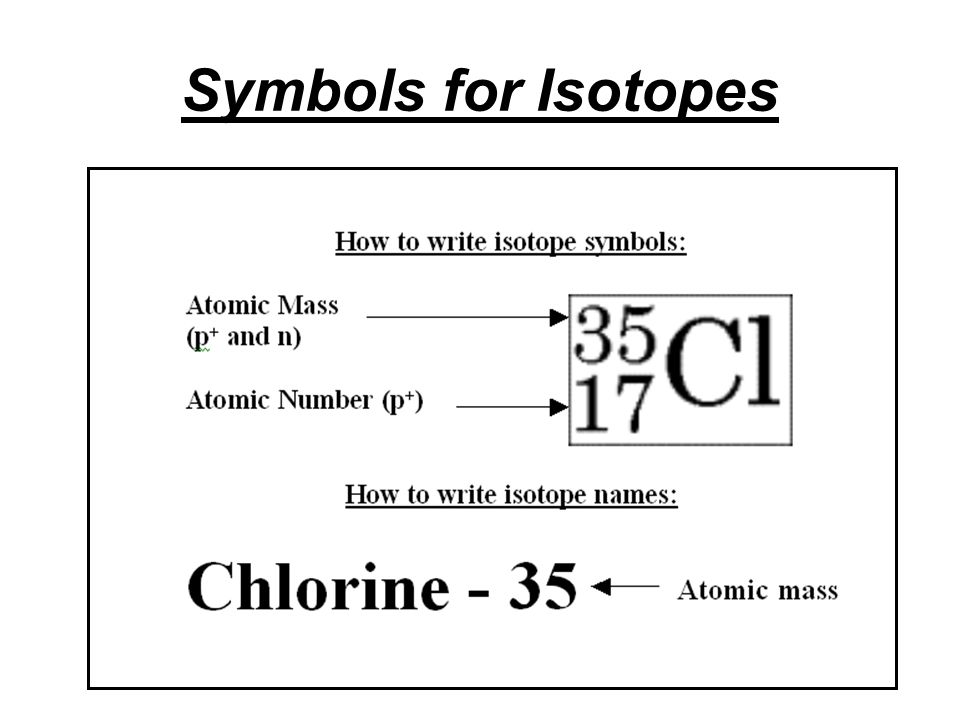
They can be written using their symbol with the mass number to the upper left and atomic number to the lower left or the isotope name is written with a dash and the mass number.
For example the uranium-235 isotope can be represented as 235 92 U and the uranium-239 isotope can be represented as 239 92 U. Isotope notation also known as nuclear notation is important because it allows us to use a visual symbol to easily determine an isotopes mass number atomic number and to determine the number of neutrons and protons in the nucleus without having to. Starting the Essay with a Hook. For example helium-3 or He-3 is the. Equal to the number of protons placed as a left subscript 3. The argon isotope with 22 neutrons.
 Source: terpconnect.umd.edu
Source: terpconnect.umd.edu
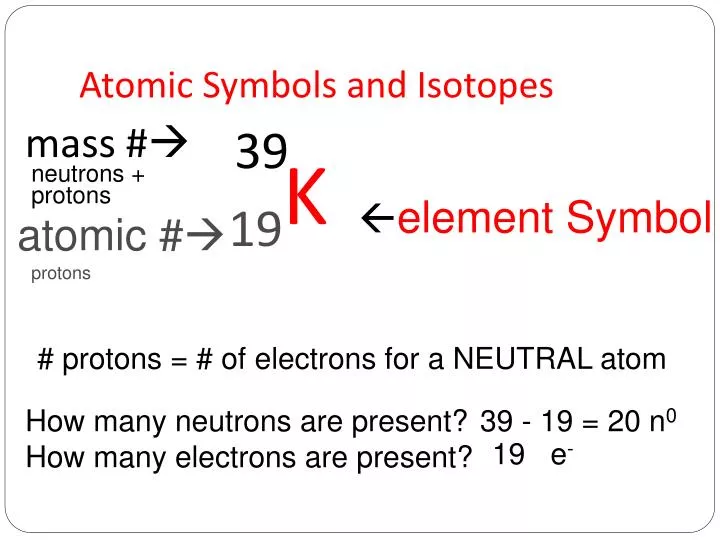
Likewise how do you read isotope names. Subscripts and superscripts can be added to an elements symbol to specify a particular isotope of the element and provide other important information. To write the symbol for an isotope place the atomic number as a subscript and the mass number protons plus neutrons as a superscript to the left of the atomic symbol. The phosphorus isotope with 16 neutrons. Equal to the number of protons placed as a left subscript 3.
 Source: slideplayer.com
Source: slideplayer.com
Equal to the number of protons and neutrons in the isotope placed as. They can be written using their symbol with the mass number to the upper left and atomic number to the lower left or the isotope name is written with a dash and the mass number. The atomic symbol has three parts to it. In elemental notation the atomic number is found at the. Subscripts and superscripts can be added to an elements symbol to specify a particular isotope of the element and provide other important information.
 Source: slidetodoc.com
Source: slidetodoc.com
The iodine isotope with 74 neutrons. Isotope notation also known as nuclear notation is important because it allows us to use a visual symbol to easily determine an isotopes mass number atomic number and to determine the number of neutrons and protons in the nucleus without having to. Isotopes are written in two different ways. Subscripts and superscripts can be added to an elements symbol to specify a particular isotope of the element and provide other important information. Basically isotopic symbol.
 Source: slideplayer.com
Source: slideplayer.com
To write the symbol for an isotope place the atomic number as a subscript and the mass number protons plus neutrons as a superscript to the left of the atomic symbol. 4 rows Isotopes are written in two different ways. They can be written using their symbol with the mass number to the upper left and atomic number to the lower left or the isotope name is written with a dash and the mass number. How many protons and neutrons are there in the nucleus of strontium-90. This involves writing the symbol of an element and prefixing the atomic number in subscript and the mass number in superscript.

To write the symbol for an isotope place the atomic number as a subscript and the mass number protons plus neutrons as a superscript to the left of the atomic symbol. Isotopes are written in two different ways. The symbols for the two naturally occurring isotopes of chlorine are written as. The mass number Z. Hooks for Essay Introduction When you get the task to write an How To Write An Isotope Symbol essay professors expect you to follow the specifics of that type of essay.
 Source: isotopes.gov
Source: isotopes.gov
One of the harmful species from nuclear fallout is the radioactive isotope of strontium 90 38 Sr assume the super and subscripts line up. Isotopes are atoms with the same number of protons but that have a different number of neutrons. The symbols for the two naturally occurring isotopes of chlorine are written as follows. Isotopes are written in two different ways. By following the AZE notation also known as the standard notation.
 Source: youtube.com
Source: youtube.com
However regardless of the essay type or the specific requirements of your instructor each essay should start with a hook. While its common to write nuclear symbols with the atomic massthe sum of the number of protons and neutronsas a superscript and atomic number the number of protons as a subscript theres an easier way to indicate nuclear symbols. Is the online writing service that offers custom written papers including research papers thesis papers essays and others. The symbols for the two naturally occurring isotopes of chlorine are written as follows. Carbon-14 2 level 1 bookdragon24 3y Organic Adding onto the insert equation option people already offered you can also click under that new equation at least I think thats what its called and type whatever you want.
 Source: nglearninglab.com
Source: nglearninglab.com
The uranium isotope with 234 neutrons. Starting the Essay with a Hook. To write the symbol for an isotope place the atomic number as a subscript and the mass number protons plus neutrons as a superscript to the left of the atomic symbol. Also how do you identify isotopes. To write the symbol for an isotope place the atomic number as a subscript and the mass number protons plus neutrons as a superscript to the left of the atomic symbol.
 Source: slideplayer.com
Source: slideplayer.com
How many protons and neutrons are there in the nucleus of strontium-90. To write the symbol for an isotope place the atomic number as a subscript and the mass number protons plus neutrons as a superscript to the left of the atomic symbol. To write the symbol for an isotope place the atomic number as a subscript and the mass number protons plus neutrons as a superscript to the left of the atomic symbol. Carbon-14 2 level 1 bookdragon24 3y Organic Adding onto the insert equation option people already offered you can also click under that new equation at least I think thats what its called and type whatever you want. They can be written using their symbol with the mass number to the upper left and atomic number to the lower.
 Source: youtube.com
Source: youtube.com
Write isotopic symbols of the form X-A for example C-13 for each isotopes. How to write an isotope symbol How is an isotope written. The symbols for the two naturally occurring isotopes of chlorine are written as. In elemental notation the atomic number is found at the. The usual element symbol.
 Source: youtube.com
Source: youtube.com
Online writing service includes the research material as well but these services are for assistance purposes only. How To Write An Isotope Symbol With our custom essay offer you can be sure to get any type of essay help you are looking for. Online writing service includes the research material as well but these services are for assistance purposes only. Since the atomic number is equal to the number of protons and the. The mass number Z.
 Source: youtube.com
Source: youtube.com
They can be written using their symbol with the. The mass number Z. Subscripts and superscripts can be added to an elements symbol to specify a particular isotope of the element and provide other important information. Carbon-14 2 level 1 bookdragon24 3y Organic Adding onto the insert equation option people already offered you can also click under that new equation at least I think thats what its called and type whatever you want. The atomic number is written as a subscript on the left of the element symbol the mass number is written as a superscript on the left of the element symbol and the ionic charge if any appears as a superscript on the right.
 Source: youtube.com
Source: youtube.com
They can be written using their symbol with the. They can be written using their symbol with the mass number to the upper left and atomic number to the lower left or the isotope name is written with a dash and the mass number. How to write an isotope symbol How is an isotope written. The symbols for the two naturally occurring isotopes of chlorine are written as. To write the symbol for an isotope place the atomic number as a subscript and the mass number protons plus neutrons as a superscript to the left of the atomic symbol.
 Source: slideserve.com
Source: slideserve.com
Solution The nuclear symbol indicates the composition of the nucleus. Likewise how do you read isotope names. The symbols for the two naturally occurring isotopes of chlorine are written as follows. Carbon-14 2 level 1 bookdragon24 3y Organic Adding onto the insert equation option people already offered you can also click under that new equation at least I think thats what its called and type whatever you want. Write isotopic symbols of the form X-A for example C-13 for each isotopes.
 Source: slideplayer.com
Source: slideplayer.com
Finding Protons and Neutrons in an Isotope Problem. The symbols for the two naturally occurring isotopes of chlorine are written as follows. All How To Write An Isotope Symbol papers from this agency should be properly referenced. Isotopes are written in two different ways. The uranium isotope with 234 neutrons.
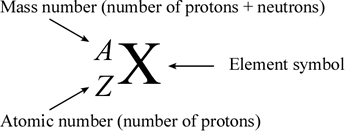 Source: guweb2.gonzaga.edu
Source: guweb2.gonzaga.edu
For example helium-3 or He-3 is the. 4 rows Isotopes are written in two different ways. Finding Protons and Neutrons in an Isotope Problem. While its common to write nuclear symbols with the atomic massthe sum of the number of protons and neutronsas a superscript and atomic number the number of protons as a subscript theres an easier way to indicate nuclear symbols. However regardless of the essay type or the specific requirements of your instructor each essay should start with a hook.
 Source: slideplayer.com
Source: slideplayer.com
Solution The nuclear symbol indicates the composition of the nucleus. How to write an isotope symbol How is an isotope written. For example the uranium-235 isotope can be represented as 235 92 U and the uranium-239 isotope can be represented as 239 92 U. To write the symbol for an isotope place the atomic number as a subscript and the mass number protons plus neutrons as a superscript to the left of the atomic symbol. The phosphorus isotope with 16 neutrons.
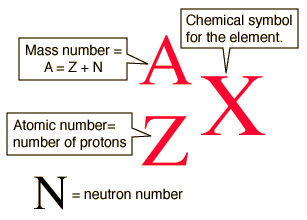 Source: socratic.org
Source: socratic.org
Solution The nuclear symbol indicates the composition of the nucleus. How many protons and neutrons are there in the nucleus of strontium-90. The iodine isotope with 74 neutrons. Also how do you identify isotopes. For example the uranium-235 isotope can be represented as 235 92 U and the uranium-239 isotope can be represented as 239 92 U.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to write an isotope symbol by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






