Your Electron dot symbol for nitrogen images are available. Electron dot symbol for nitrogen are a topic that is being searched for and liked by netizens now. You can Download the Electron dot symbol for nitrogen files here. Download all royalty-free photos and vectors.
If you’re searching for electron dot symbol for nitrogen images information linked to the electron dot symbol for nitrogen interest, you have pay a visit to the ideal site. Our site frequently provides you with hints for seeking the maximum quality video and image content, please kindly surf and find more enlightening video articles and graphics that fit your interests.
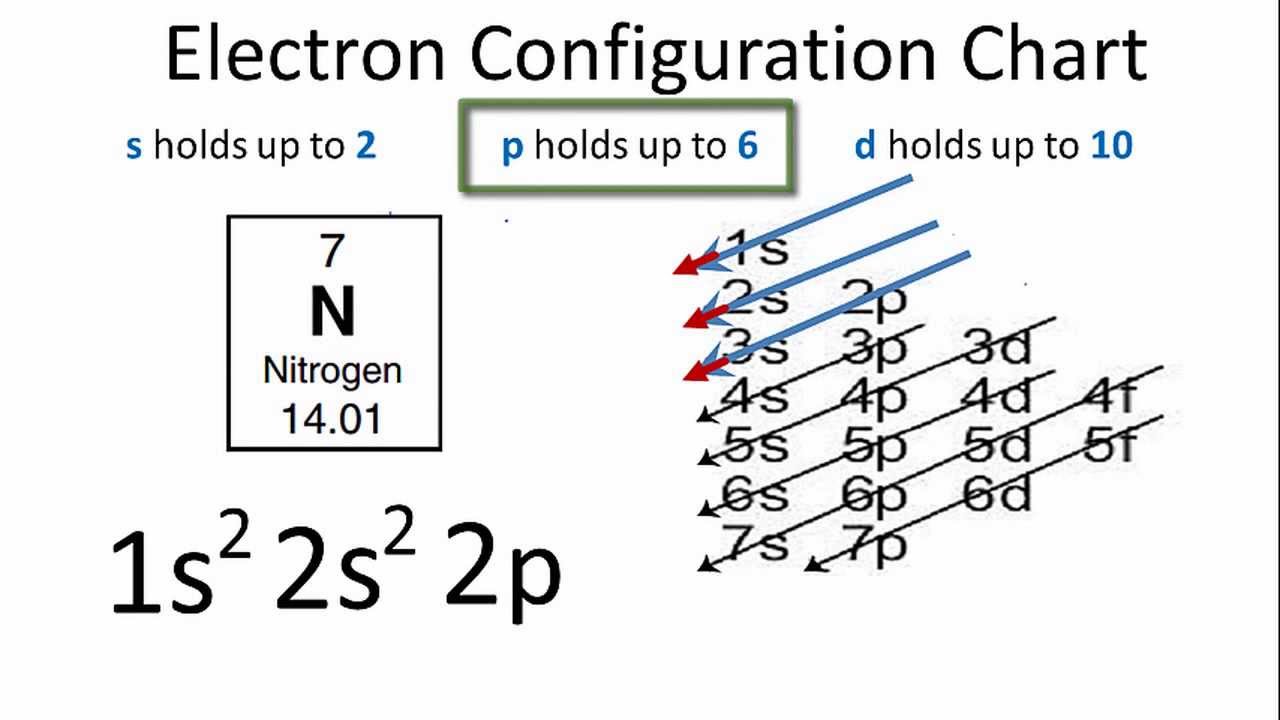
Electron Dot Symbol For Nitrogen. Since it is in Group 5 it will have 5 valence electrons. Nitrogen Gas Diatomic Nitrogen - YouTube. Nitrogen has five valence electrons. How to Draw the Lewis Dot Structure for N2.
 Gcse Chemistry The Reaction Between Sodium And Chlorine Balanced Chemical Equation What Is An Ionic Bond Why Ionic Bonding Chemical Equation Chemistry From pinterest.com
Gcse Chemistry The Reaction Between Sodium And Chlorine Balanced Chemical Equation What Is An Ionic Bond Why Ionic Bonding Chemical Equation Chemistry From pinterest.com
Nitrogen is in Group 5 sometimes called Group V or Group 15. The electrons in the valence shell are shown as dots placed around the symbol. Can be used as content for research and analysis. The number of dots corresponds toan. Nitrogen Gas Diatomic Nitrogen - YouTube. A Lewis Electron Dot Formula comprises one dot for every valence electron and an elements symbol.
Electron Dot Diagrams Noble Gases We learned that the noble gases have 8.
Note down a skeletal structure displaying a realistic bonding pattern by means of only the element symbols. The two lone nonbonding electron pairs one on each N atom lie at a Apr 18 2015. Write electron dot symbols for the following atoms. A step-by-step explanation of how to draw the Lewis dot structure for N Nitrogen. For example we learned that chlorine has 7 valence electrons. Electron Dot Diagrams Noble Gases We learned that the noble gases have 8.
 Source: pinterest.com
Source: pinterest.com
The two lone nonbonding electron pairs one on each N atom lie at a Apr 18 2015. Collected from the entire web and summarized to include only the most important parts of it. Pick up every valence electrons from every atom and toss them into a make-believe container. Can be used as content for research and analysis. When nitrogen gains three electrons it forms the nitride N 3- ion.
 Source: in.pinterest.com
Source: in.pinterest.com
Search only database of. Since it is in Group 5 it will have 5 valence electrons. Nitrogen Dioxide NO 2 is a covalent compound that is composed of a central nitrogen atom single bonded to an oxygen atom and a double bond with another oxygen atom. There are also two more valence electrons and they are paired with two of the. There are now four unpaired electrons around the oxygen symbol.
 Source: pinterest.com
Source: pinterest.com
Search only database of. Write symbols for the ions formed by the following Gain of 1 electron by Chlorine Gain of 3 electrons by Arsenic Loss. Boron which also has three unpaired valence electrons in its Lewis dot symbol also tends to form compounds with three bonds whereas carbon with four unpaired valence electrons in its. There are now four unpaired electrons around the oxygen symbol. Electron Dot Diagrams Noble Gases We learned that the noble gases have 8.
 Source: in.pinterest.com
Source: in.pinterest.com
Oxygen is in group 16VIA so it has six valence electrons. The electron dot diagram for an element shows the valence electrons for the element. Electron Dot Diagrams Noble Gases We learned that the noble gases have 8. For example we learned that chlorine has 7 valence electrons. Advanced searches left.
 Source: pinterest.com
Source: pinterest.com
There are now four unpaired electrons around the oxygen symbol. The two lone nonbonding electron pairs one on each N atom lie at a Apr 18 2015. Nitrogen is in Group 5 sometimes called Group V or Group 15. Nitrogen Dioxide NO 2 is a covalent compound that is composed of a central nitrogen atom single bonded to an oxygen atom and a double bond with another oxygen atom. Since it is in Group 5 it will have 5 valence electrons.
 Source: pinterest.com
Source: pinterest.com
At room temperatures nitrogen dioxide is a reddish-brown gas that has a density of 18 gdm 3. How to Draw the Lewis Dot Structure for N2. The electron dot symbol for chlorine is shown at the right. Click to see full answer Considering this what are electron dot diagrams used for. I show you where Nitrogen is on the periodic table and how to determine.
 Source: pinterest.com
Source: pinterest.com
Nitrogen has five valence electrons. Can be used as content for research and analysis. Collected from the entire web and summarized to include only the most important parts of it. Search only database of. The electron dot symbol for chlorine is shown at the right.
 Source: pinterest.com
Source: pinterest.com
Boron which also has three unpaired valence electrons in its Lewis dot symbol also tends to form compounds with three bonds whereas carbon with four unpaired valence electrons in its Lewis dot symbol. Electron Dot Diagram For Nitrogen. Since it is in Group 5 it will have 5 valence electrons. Dinitrogen N 2 refers to nitrogen occurring in the diatomic form its most stable form also commonly called molecular nitrogen or nitrogen gas in which two nitrogen atoms are linked by a triple covalent bond which fills the Lewis electron rules. The two lone nonbonding electron pairs one on each N atom lie at a Apr 18 2015.
 Source: pinterest.com
Source: pinterest.com
At room temperatures nitrogen dioxide is a reddish-brown gas that has a density of 18 gdm 3. Click to see full answer Considering this what are electron dot diagrams used for. There are now four unpaired electrons around the oxygen symbol. The Lewis dot structure of a nitrogen atom would be the capitolletter N with the five valence electrons represented by two dotsabove it one to the left right and bottom of it. If you are looking at a nitrogen atom by itself it will have 5electrons dots surrounding it.
 Source: pinterest.com
Source: pinterest.com
Click to see full answer Considering this what are electron dot diagrams used for. The electron dot formula for nitrogen looks like this with five dots. NO2 Nitrogen Dioxide Lewis Dot Structure. I show you where Nitrogen is on the periodic table and how to determine. The two lone nonbonding electron pairs one on each N atom lie at a Apr 18 2015.
 Source: pinterest.com
Source: pinterest.com
If you are looking at a nitrogen atom by itself it will have 5electrons dots surrounding it. Pick up every valence electrons from every atom and toss them into a make-believe container. It needs three electrons to complete the octet for 2s2p subshells. When nitrogen gains three electrons it forms the nitride N 3- ion. Click to see full answer Considering this what are electron dot diagrams used for.
 Source: pinterest.com
Source: pinterest.com
If you are looking at a nitrogen atom by itself it will have 5electrons dots surrounding it. When you draw the Lewis structure for Nitrogen youll put five dots or valance electrons around the element symbol N. A Lewis Electron Dot Formula comprises one dot for every valence electron and an elements symbol. Write symbols for the ions formed by the following Gain of 1 electron by Chlorine Gain of 3 electrons by Arsenic Loss. Nitrogen Gas Diatomic Nitrogen - YouTube.
 Source: pinterest.com
Source: pinterest.com
Iodine Selenium Strontium Nitrogen4-2. Stages to articulate the electron dot formula are stated beneath. Home Blog Pro Plans B2B solution Login. The two lone nonbonding electron pairs one on each N atom lie at a Apr 18 2015. Pick up every valence electrons from every atom and toss them into a make-believe container.
 Source: pinterest.com
Source: pinterest.com
Boron which also has three unpaired valence electrons in its Lewis dot symbol also tends to form compounds with three bonds whereas carbon with four unpaired valence electrons in its Lewis dot symbol. A step-by-step explanation of how to draw the Lewis dot structure for N Nitrogen. When nitrogen gains three electrons it forms the nitride N 3- ion. Nitrogen Dioxide NO 2 is a covalent compound that is composed of a central nitrogen atom single bonded to an oxygen atom and a double bond with another oxygen atom. Electron Dot Diagram For Nitrogen.
 Source: pinterest.com
Source: pinterest.com
Note down a skeletal structure displaying a realistic bonding pattern by means of only the element symbols. Nitrogen is in Group 5 sometimes called Group V or Group 15. The Lewis dot symbol explains why nitrogen with three unpaired valence electrons tends to form compounds in which it shares the unpaired electrons to form three bonds. Can be used as content for research and analysis. Pick up every valence electrons from every atom and toss them into a make-believe container.
 Source: pinterest.com
Source: pinterest.com
Nitrogen has five valence electrons. Advanced searches left. The number of dots corresponds toan. Boron which also has three unpaired valence electrons in its Lewis dot symbol also tends to form compounds with three bonds whereas carbon with four unpaired valence electrons in its Lewis dot symbol. Nitrogen Gas Diatomic Nitrogen - YouTube.
 Source: pinterest.com
Source: pinterest.com
Note down a skeletal structure displaying a realistic bonding pattern by means of only the element symbols. The electron dot formula for nitrogen looks like this with five dots. This is explained by the fact that nitrogen has the electron configuration 1s 2 2s 2 2p 3. The Lewis dot structure of a nitrogen atom would be the capitolletter N with the five valence electrons represented by two dotsabove it one to the left right and bottom of it. A Lewis Electron Dot Formula comprises one dot for every valence electron and an elements symbol.
 Source: pinterest.com
Source: pinterest.com
Iodine Selenium Strontium Nitrogen4-2. Boron which also has three unpaired valence electrons in its Lewis dot symbol also tends to form compounds with three bonds whereas carbon with four unpaired valence electrons in its Lewis dot symbol. Lithium 1 s 2 2 s 1 1 valence electron nitrogen 1 s 2 2 s 2 2 p 3 5 valence electrons neon 1 s 2 2 s 2 2 p 6 8 valence electrons. When nitrogen gains three electrons it forms the nitride N 3- ion. Group 13 has three valence electrons Group 14 has four up through Group 18 with eight.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title electron dot symbol for nitrogen by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






